 Abstract.
Both sexes of the bark beetle Dendroctonus brevicomis convert the (+)
and (-) enantiomers of the tree terpene alpha-pinene to the corresponding enantiomers
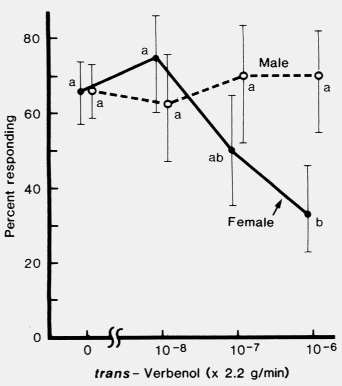
of trans-verbenol at about equal rates. (-)-trans-Verbenol inhibited the response of'
females, but not of males, to a mixture of attractive pheromone components. Since
the female initiates the attack on a pine tree, (-)-trans-verbenol may play a role in
reducing intraspecific competition for breeding areas.
Abstract.
Both sexes of the bark beetle Dendroctonus brevicomis convert the (+)
and (-) enantiomers of the tree terpene alpha-pinene to the corresponding enantiomers
of trans-verbenol at about equal rates. (-)-trans-Verbenol inhibited the response of'
females, but not of males, to a mixture of attractive pheromone components. Since
the female initiates the attack on a pine tree, (-)-trans-verbenol may play a role in
reducing intraspecific competition for breeding areas.
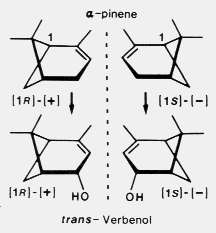 In contrast, I found that both
sexes of D. brevicomis use both enantiomers of alpha-pinene to synthesize the corresponding
enantiomers of trans-verbenol (12) and synthesize only trace amounts,
if any, of cis-verbenol.
In contrast, I found that both
sexes of D. brevicomis use both enantiomers of alpha-pinene to synthesize the corresponding
enantiomers of trans-verbenol (12) and synthesize only trace amounts,
if any, of cis-verbenol.| Table 1. Amounts of trans-verbenol, verbenone, and myrtenol in hindguts of male and female D. brevicomis when exposed or not exposed to vapors of each enantiomer of alpha-pinene. There were no significant differences between males and females in the amount of trans-verbenol or myrtenol produced from each enantiomer of alpha-pinene or in the amounts of trans-verbenol or myrtenol produced from each enantiomer by either sex (P > 0.05). Significantly more trans-verbenol and myrtenol were produccd in males and females exposed to enantiomers of alpha-pinene than in controls not exposed to vapor for 18 hours (P < 0.001). | |||
| Production per beetle (x 10-7g) | |||
|---|---|---|---|
| Treatment | trans-Verbenol | Verbenone | Myrtenol |
| Male | |||
| (+)-alpha-pinene* | 58.5 ± 9.8 | 6.4 ± 1.5 | 20.5 ± 2.9 |
| (+)-alpha-pinene§ | 135.3 ± 4.8 | 13.3 ± 2.7 | 44.9 ± 4.8 |
| (-)-alpha-pinene* | 74.6 ± 10.8 | 12.4 ± 0.6 | 10.7 ± 1.5 |
| (-)-alpha-pinene§ | 218.1 ± 20.6 | 22.9 ± 5.1 | 28.6 ± 3.4 |
| None¥ | 0.5 ± 0.1 | 6.2 ± 2.4 | 0.2 ± 0.1 |
| No vapor (18 hours)¥ | 3.0 ± 0.5$ | 27.9 ± 3.6 | 0.8 ± 0.3 |
| Female | |||
| (+)-alpha-pinene* | 65.9 ± 13.9 | < 0.1$ | 29.0 ± 6.4 |
| (+)-alpha-pinene§ | 139.9 ± 16.7 | < 0.1 | 45.2 ± 5.9 |
| (-)-alpha-pinene* | 89.7 ± 9.4 | < 0.1 | 17.7 ± 1.4 |
| (-)-alpha-pinene§ | 208.8 ± 28.9 | < 0.1 | 37.6 ± 5.5 |
| None¥ | 0.7 ± 0.1 | < 0.1 | 0.1 ± 0.1 |
| No vapor (18 hours)¥ | 6.6 ± 1.3$ | < 0.1 | 0.6 ± 0.1 |