 Ips paraconfusus bark beetles were allowed to fed and bore in a powdered-cellulose based diet with or without antibiotics.
Ips paraconfusus bark beetles were allowed to fed and bore in a powdered-cellulose based diet with or without antibiotics.
 The bacterium Bacillus cereus isolated
from hindguts of male and female Ips paraconfusus (Coleoptera: Scolytidae)
produced the pheromone cis-verbenol
when exposed in culture to vapors of alpha-pinene, a terpene found in the host,
ponderosa pine (1). Exposure of male
and female beetles to (-)-alpha-pinene vapor
resulted in the production of cis-verbenol and myrtenol in the hindgut,
indicating a relation between precursor and
product (2). Another host plant chemical, myrcene, was shown to be convert-
ed to the pheromones ipsenol and ipsdienol, but only in male I. paraconfusus
(3-5). Therefore, we added antibiotics to
the diets of male I. paraconfusus and
then exposed the insects to vapors of
(-)-alpha-pinene and myrcene to determine
if pheromone synthesis could be inhibited,
thereby implicating symbiotic microorganisms.
The bacterium Bacillus cereus isolated
from hindguts of male and female Ips paraconfusus (Coleoptera: Scolytidae)
produced the pheromone cis-verbenol
when exposed in culture to vapors of alpha-pinene, a terpene found in the host,
ponderosa pine (1). Exposure of male
and female beetles to (-)-alpha-pinene vapor
resulted in the production of cis-verbenol and myrtenol in the hindgut,
indicating a relation between precursor and
product (2). Another host plant chemical, myrcene, was shown to be convert-
ed to the pheromones ipsenol and ipsdienol, but only in male I. paraconfusus
(3-5). Therefore, we added antibiotics to
the diets of male I. paraconfusus and
then exposed the insects to vapors of
(-)-alpha-pinene and myrcene to determine
if pheromone synthesis could be inhibited,
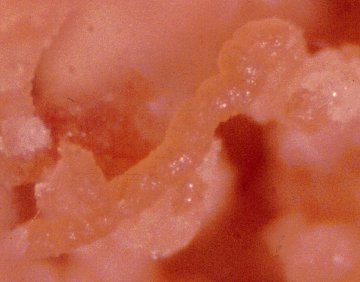
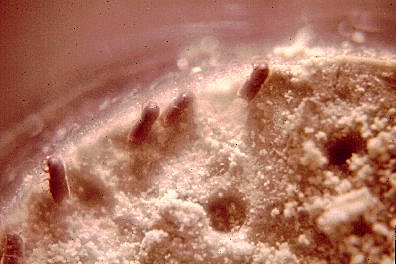
thereby implicating symbiotic microorganisms. Side view of petri dish with powdered-cellulose based diet containing phloem particles
and with or without antibiotics. Note the tunnels of Ips paraconfusus bark beetles along the inner wall
of petri dish.
Side view of petri dish with powdered-cellulose based diet containing phloem particles
and with or without antibiotics. Note the tunnels of Ips paraconfusus bark beetles along the inner wall
of petri dish.
| Table 1. Pheromones and metabolites produced in male I. paraconfusns exposed to myrcene and (-)-alpha-pinene vapors after being fed diets with and without streptomycin sulfate or penicillin G (or a combination thereof) at 4.1 mg per milliliter of diet. In experiment 1, the beetles were exposed to myrcene at 21.1 ± 1.2 x 10-7g/ml and to (-)-alpha-pinene at 47.1 ± 2.8 x 10-7g/ml. In experiment 2, beetles were exposed to myrcene at 17.6 ± 0.6 x 10-7g/ml and to (-)-alpha-pinene at 51.0 ± 3.0 x 10-7g/ml. | |||||
| Mean amount of pheromone per male (10-8 g ± S.E.M.) | |||||
|---|---|---|---|---|---|
| Treatment | Ipsdienol | Ipsenol | cis-Verbenol*¥ | Myrtenol¥ | Compound B¥ ¤ |
| Experiment 1 | |||||
| No antibiotic | 3.9 ± 0.6 | 9.5 ± 1.8 | 294.2 ± 40.0 | 96.0 ± 12.5 | 609.8 ± 42.7 |
| Streptomycin and penicillin G | < 0.1§ | < 0.1§ | 345.8 ± 51.7 | 123.5 ± 15.0 | 467.4 ± 44.2 |
| Experiment 2 | |||||
| No antibiotic | 6.2 ± 1.8¥ | 45.1 ± 8.8¥ | 1179.4 ± 228.7 | 379.3 ± 66.5 | 1123.0 ± 106.0 |
| Streptomycin | < 0.5§ | < 0.5§ | 1098.6 ± 195.6 | 351.5 ± 72.4 | 996.6 ± 55.7 |
| Penicillin G | 3.6 ± 0.7¥ | 26.3 ± 5.2¥ | 1145.5 ± 182.9 | 380.1 ± 27.6 | 991.4 ± 76.9 |
| Unfed | 3.4 ± 0.8¥ | 24.4 ± 5.0¥ | 998.3 ± 142.4 | 335.6 ± 38.7 | 1208.1 ± 180.6 |
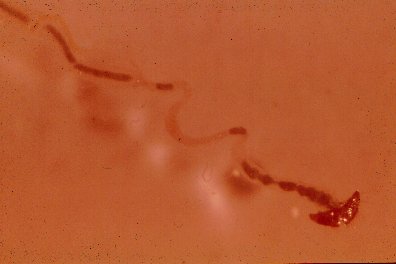 Mid- and hindgut of Ips paraconfusus containing cellulose-phloem diet with or without antibiotics.
Mid- and hindgut of Ips paraconfusus containing cellulose-phloem diet with or without antibiotics.