Byers, J.A., Zhang, Q.H., Schlyter, F. and Birgersson, G. 1998. Volatiles from
Nonhost Birch Trees Inhibit Pheromone Response in Spruce Bark Beetles.
Naturwissenschaften 85:557-561.  pdf
pdf

Volatiles from nonhost birch bark, Betula pendula, inhibit the orientation response
of bark beetles, Pityogenes chalcographus and Ips typographus (Coleoptera:
Scolytidae), to their aggregation pheromone components. Odors from leaves of B.
pubescens also reduces attraction of both species. Neither species colonized birch trees
baited with synthetic pheromones while readily accepting their host Norway spruce, Picea
abies. (Z)-3-hexen-1-ol and 1-hexanol were found in air drawn from birch leaves,
and both compounds reduce attraction of these beetles to their pheromone. Bark beetles may
have evolved these behaviors to avoid wasting time investigating or boring in nonhost trees.
 The bark beetles I. typographus (at left) and P. chalcographus are important
enemies of Norway spruce, P. abies, the dominant tree of northern European forests
[Austarå et al. 1984]. These beetles must find a susceptible host tree and then aggregate in
large numbers by attraction to a pheromone in order to overpower the resinous defenses of
the tree [Byers 1995, 1996]. The host finding phase of bark beetles is the most risky period
of the life cycle, with mortality up to 80 percent or more as judged by ratios of emergence to
entrance hole numbers in bark samples [Byers 1996]. Thus, it would be advantageous for bark
beetles to find their host tree as quickly as possible. Natural selection may have caused bark
beetles to evolve several mechanisms for finding their host tree as well as for avoiding
unsuitable host and nonhost species of plants. Host selection has long been hypothesized to
be a complex process comprising both affinity for host chemicals and avoidance of nonhost
chemicals [Thorsteinson 1960].
The bark beetles I. typographus (at left) and P. chalcographus are important
enemies of Norway spruce, P. abies, the dominant tree of northern European forests
[Austarå et al. 1984]. These beetles must find a susceptible host tree and then aggregate in
large numbers by attraction to a pheromone in order to overpower the resinous defenses of
the tree [Byers 1995, 1996]. The host finding phase of bark beetles is the most risky period
of the life cycle, with mortality up to 80 percent or more as judged by ratios of emergence to
entrance hole numbers in bark samples [Byers 1996]. Thus, it would be advantageous for bark
beetles to find their host tree as quickly as possible. Natural selection may have caused bark
beetles to evolve several mechanisms for finding their host tree as well as for avoiding
unsuitable host and nonhost species of plants. Host selection has long been hypothesized to
be a complex process comprising both affinity for host chemicals and avoidance of nonhost
chemicals [Thorsteinson 1960].
Many of the most `aggressive' tree-killing bark beetles are believed to initially find
host trees by landing at random since there is no evidence of a long-range attraction to
uncolonized hosts [Moeck et al. 1981; Byers 1995, 1996]. Most of these bark beetle species
exhibit reduced attraction to pheromone in the presence of unsuitable hosts that are decayed
or fully colonized and releasing ethanol and verbenone [Bakke 1981; Klimetzek et al. 1986;
Byers et al. 1989; Schlyter et al. 1989; Schroeder and Lindelöw 1989; Byers 1992, 1993,
1995]. In addition, studies with the North American species, Dendroctonus frontalis,
I. grandicollis and I. avulsus, indicated that bark beetles might avoid nonhost
trees because their response to pheromone was inhibited by several compounds among the
`green-leaf volatiles' [Dickens et al. 1991, 1992]. In Europe, the weak attraction to ethanol,
believed released from damaged Scots pine hosts, by Tomicus piniperda [Schroeder
and Lindelöw 1989; Byers 1992] was shown to be reduced by odors from logs of nonhosts
aspen (Populus tremula) and birch (B. pendula) [Schroeder 1992]. Following
this, the aggregation responses of I. typographus and I. duplicatus to
pheromone, and T. piniperda to host monoterpenes, were shown to be inhibited by a
blend of six green-leaf volatiles [Schlyter et al. 1995]. Recently, the responses of North
American D. ponderosae and Trypodendron lineatum to pheromone components
were reduced by (Z)-3-hexen-1-ol and other green-leaf volatiles [Wilson et al. 1996;
Borden et al. 1997].
Two species of birch, B. pendula and B. pubescens, are the most
common deciduous trees in Norway spruce forests of Scandinavia. To determine whether
spruce bark beetles, I. typographus and P. chalcographus, may avoid the
nonhost birch, pheromone baits were placed in a trap-pair rotor that separated the baits apart
by 6 m at 1.2 m height and revolved at 2 rph [Byers et al. 1990]. The traps consisted of plastic
cylinders (18 cm diam. x 28 cm high) covered at the top but open at the bottom and
suspended over a funnel (31 cm diam.) that collected beetles striking the cylinder (figure 1).
Appropriate pheromone baits [Bakke et al. 1977; Francke et al. 1977; Byers et al. 1988, 1990]
were placed inside the cylinders that also contained either a fine screen cage or one
containing freshly cut birch bark chips or leaves (figure 1). (Z)-3-hexen-1-ol was tested
similarly with pheromone baits since it is a known volatile from birch, B. pendula [König
et al. 1995], and is also active in reducing aggregation of D. ponderosa and T.
lineatum in North America [Wilson et al. 1996; Borden et al. 1997]. 1-Hexanol was tested
because it disrupts pheromone response in other bark beetles [Dickens et al. 1991, 1992] as
well as being found earlier in hindguts of male P. chalcographus [Francke et al. 1977].
The tests showed that volatiles from birch bark and leaves inhibited the attraction
of both I. typographus and P. chalcographus to their synthetic pheromones
(figure 1).
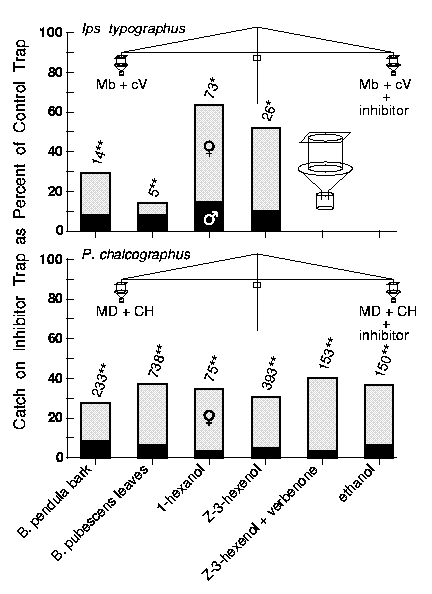
Figure 1. Reduction of catch of Ips typographus and Pityogenes chalcographus on
traps releasing pheromone plus inhibitor volatiles compared to a control pheromone trap. The trap pairs
were mechanically-rotated at 2 rph to minimize catch variation due to trap position. Test replicates were
conducted for at least 1 hour, and after each replicate the inhibitory source, but not the attractants, was
switched to the other trap. Replicate catches were summed and the paired control and treatment were
compared with a Chi square goodness of fit test to an expected catch if there were no differences
based on the average for both traps. About 100 g leaves and 400 g bark chips were used in various
tests during June (1997 and 1998), Torsby, Sweden. I. typographus pheromone components,
MB (2-methyl-3-butenol) and cV (cis-verbenol), were released at 50 mg and 1 mg/day,
respectively. (Z)-3-hexen-1-ol and 1-hexanol (both 98%, Aldrich), verbenone ([alpha]20D= -246°,
99.2% e.e., Bedoukian), and ethanol were released at 8.6, 5.7, 1, and 800 mg/day, respectively. P.
chalcographus pheromone components, MD (E,Z-2,4-methyl decadienoate, > 99.5%)
and CH (chalcogran, 46% E:54% Z, > 98%), were released at 70 µg and 0.8 mg per day, respectively.
Catches, as signified by numbers above the bars, were significantly different between baits in the same
test when designated with * or ** indicating P < 0.01 or 0.001, respectively (Chi square goodness of
fit).
The green-leaf volatiles, (Z)-3-hexen-1-ol and 1-hexanol, also reduced
attraction in both bark beetle species (figure 1). Males of P. chalcographus were
inhibited more so than females by odors from birch leaves or the two green-leaf volatiles since
male percentages of catch (9% to 17.5%) were significantly lower than on the pheromone
controls (19.5% to 26%; P<0.001 in all cases). No differences in catch between the sexes
were observed due to bark volatiles or in any comparison with I. typographus.
The addition of verbenone, a known inhibitor of P. chalcographus [Byers
1993], further reduced the inhibition of P. chalcographus by (Z)-3-hexen-1-ol
(figure 1), indicating a synergism between the two inhibitors. Verbenone plus a blend of six
green-leaf compounds have earlier been indicated as being synergistic in reducing response
in Tomicus piniperda and I. typographus [Schlyter et al. 1995]. Ethanol at 800
mg/day, a relatively high release rate [Schroeder and Lindelöw 1989; Byers 1992], reduced
the attraction of P. chalcographus to pheromone (figure 1). This is in accordance with
the theory that ethanol from decadent hosts reduces response of `primary' bark beetles such
as I. typographus [Klimetzek et al. 1986] that colonize living trees that release little or
no ethanol [Moeck 1970; Kimmerer and Kozlowski 1982].
To determine if (Z)-3-hexen-1-ol, 1-hexanol or other compounds are present
in odor from birch, volatiles were collected from bark of B. pendula and shoots with
leaves of B. pubescens from the same trees as used in the field experiments, as well
as from leaves of both species from Lomma (near Malmö), Sweden. The uncut ends of the
shoots with leaves or bark chips were enclosed in plastic cooking bags (Meny® Toppits®,
35x43 cm) through which activated-carbon filtered air was sucked at 150 ml/min. The effluent
volatiles were adsorbed on 30 mg Porapak Q (50-80 mesh, Supelco) in a 3 mm ID Teflon tube
for 2.5 h. Diethyl ether washings (300 µl) of the Porapak Q were kept at -20ºC until chemical
analysis on a combined HP 5890 series II gas chromatograph and HP 5972 mass selective
detector (GC-MSD, see figure 2).
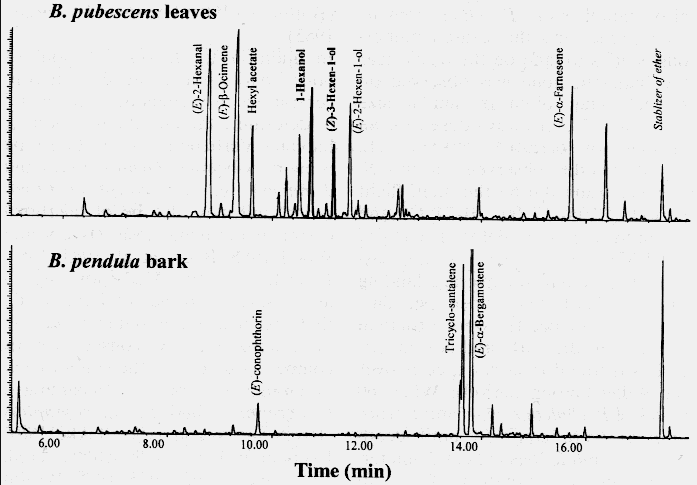
Figure 2. Upper gas chromatogram (GC) of volatiles collected from air passed over leaves on
terminal branches of Betula pubescens (cut 27 June 1997 and given water until ends of leafy
shoots were placed in bags for volatile collection on 30 June 1997 at 27ø C). Lower chromatogram of
volatiles from air passed over bark chips of B. pendula (chips cut from tree on 27 June 1997 and
held at 5ø C until placed in bags for volatile collection 30 June 1997; leaves and bark from Torsby,
Sweden). A fused-silica column, 0.25 mm x 25 m, coated with CP-wax 58 (PEG, 0.5 µm) CB was used
for GC with helium as the carrier gas at a constant flow of 31 cm/s on a temperature program of 30º
C for 3 min, a 10º C/min rise to 200º, and then constant for 10 min. Chemicals were identified by
comparison of retention times and mass spectra to those of authentic compounds and computer data
libraries (NBS75K and KEM-EKOL).
The analyses showed that (Z)-3-hexen-1-ol and 1-hexanol are major
constituents of the birch-leaf volatiles from both B. pubescens (figure 2) and B.
pendula (not shown, but about 27% and 19%, respectively, as much as B.
pubescens from Lomma, Sweden). However, these compounds were not present in
detectable amounts in bark odor (figure 2). (Z)-3-hexen-1-ol and 1-hexanol were
collected from B. pubescens leaves from Torsby, Sweden, at 1.2 and 2.64 mg/day/100
g fresh weight which should be similar to the release rates from the leaves used in the field
traps (figure 1). Thus, these two compounds were released from the leaves in the field
experiments at about 10% and 46% of the release rates for the neat compounds in the rotor
traps, respectively. This means that the inhibition of both bark beetle species by birch-leaf
volatiles is due in part to (Z)-3-hexen-1-ol and 1-hexanol. However, the reduced
attraction to pheromone in both species by odors of birch bark probably cannot be explained
by these two compounds as they were not detected in odor collections.
Interestingly, we found (E)-conophthorin, a spiroacetal, is released in
significant amounts from bark of both birch species, but not from leaves (figure 2) or from
Norway spruce bark. (E)-conophthorin has previously been identified in European ash
bark beetles (Leperisinus varius) and North American cone beetles (Conophthorus
resinosae and C. coniperda) and inhibits attraction of these beetles to pheromones
[Kohnle et al. 1992; Birgersson et al. 1995; Pierce et al. 1995]. Conophthorin is also present
in bark of North American B. papyrifera and several other deciduous trees and inhibits
aggregation of two conifer bark beetles (D. ponderosae and D. pseudotsugae)
of North America (J.H. Borden, personal communication). 1-Hexanol, found only in male P.
chalcographus hindguts during feeding [Francke et al. 1977; Byers et al. 1990], may be
a multifunctional semiochemical informing beetles of (1) likely competition at close-range on
the host or (2) an unsuitable birch tree.
Nonhost trees (B. pendula and Pinus sylvestris of about 35 cm DBH)
baited with synthetic pheromone of I. typographus (as in figure 1) attracted beetles but
few bored in, and then only a few mm, compared with hundreds of galleries in host spruce
trees similarly baited (10-14 June 1997). Two smaller birch, B. pendula and B.
pubescens, (14 cm DBH) were also baited with P. chalcographus pheromone and
this caused numerous beetles to land on the trunk near the bait, but none were observed on
several other birch of similar size standing 1.5 to 3 m away. Based on an average of 6.2±
0.9 (± 95% CL, N=20) beetles observed on the B. pendula trunk during the main flight
period (12:00-17:00, 20-24º C) and on an average time before leaving of 121± 57 s (± 95% CL,
N=20), assumed to be half the average landing time, then at least 460 landed per day.
However, no beetles were observed to bite into the bark and not one attack was found in
either species over the four-day baiting periods. When 60 males of P. chalcographus
and I. typographus were confined until death with B. pendula logs (12 cm diam.
x 28 cm) in the laboratory, 37% and 23% bored in, respectively, but only in the rough bark
areas, and then only a few mm into the phloem. No breeding galleries resulted after addition
of an equal number of females 24 hours later compared with Norway spruce logs, where
nearly all beetles made egg galleries.
The avoidance of odors of birch bark and green-leaf volatiles by I.
typographus and P. chalcographus before landing in the experiments could mean
that these bark beetles avoid flying into areas with nonhost trees. However, this question
cannot be answered until volatile concentrations in the forest some distance from birch trees
are measured. In mixed birch and spruce stands, and at some distance, the mixing of odors
would not seem to provide a useful signal. Nevertheless, after landing on a birch tree, the
concentration of volatiles at the bark surface is probably sufficient to induce beetles to soon
leave, as observed in the experiments with pheromone-baited birch trees. Beetles that can
recognize the nonhost more rapidly would be favored in evolution since they would save
minutes to hours of time that otherwise would be needed to bore into the bark and taste, all
the time being exposed to enemies. The fitness benefits of a quick decision concerning host
suitability after landing is obvious since bark beetles exhaust their fat reserves after only a few
hours of flight [Botterweg 1982; Forsse and Solbreck 1985].
In addition to the use of nonhost odors, bark beetles can discern concentrations
of verbenone and ethanol to determine the unsuitability of bark habitat. Verbenone is produced
by some bark beetles (Dendroctonus and Tomicus), but not by Ips or
Pityogenes, and may also be of microbial origin in decaying hosts [Byers and Wood
1980; Byers et al. 1984, 1989, 1990; Byers 1995]. Verbenone signifies to several species of
bark beetle that the host is not suitable because of colonization by conspecifics or competing
species in the genera Dendroctonus, Tomicus, Ips (including I.
typographus), and P. chalcographus [Byers and Wood 1980; Bakke 1981; Byers
et al. 1984, 1989; Schlyter et al. 1989; Byers 1992, 1993, 1995; Wilson et al. 1996; Borden
et al. 1997]. Ethanol also indicates microbial fermentation and unsuitable habitat to be avoided
for species colonizing living trees [Moeck 1970; Kimmerer and Kozlowski 1982; Klimetzek et
al. 1986; Schroeder and Lindelöw 1989; Byers 1992]. It is becoming apparent that bark
beetles have a more complex chemical vocabulary than previously believed to aid them in
their selection of host trees and gallery sites.
JOHN A. BYERS1, QING-HE ZHANG1, FREDRIK SCHLYTER1, and GÖRAN BIRGERSSON2
1Department of Plant Protection, Swedish University of Agricultural Sciences, SE-230 53 Alnarp, Sweden
2Chemical Ecology, Göteborg University, SE-405 30 Göteborg, Sweden
|
|---|
REFERENCES
Austarå, O., Annila, E., Bejer, B., Ehnström, B. (1984) Insect pests in forests of the
Nordic countries 1977-1981. Fauna Norv. Ser. B. 31:8-15
Bakke, A. (1981) Inhibition of the response in Ips typographus to the aggregation
pheromone:Field evaluation of verbenone and ipsenol. Z. Angew. Entomol. 92:172-177
Bakke, A., Fríyen, P., Skattebíl, L. (1977) Field response to a new pheromonal
compound isolated from Ips typographus. Naturwissenschaften 64:98.
Birgersson, G., DeBarr, G.L., de Groot, P., Dalusky, M.J., Pierce, H.D.Jr., Borden, J.H.,
Meyer, H., Francke, W., Espelie, K.E., Berisford, C.W. (1995) Pheromones in white
pine cone beetle, Conophthorus coniperda (Schwarz) (Coleoptera:
Scolytidae). J. Chem. Ecol. 21:143-167
Borden, J. H., Chong, L. J., Savoie, A., Wilson, I. M. (1997) Responses to green leaf
volatiles in two biogeoclimatic zones by striped ambrosia beetle, Trypodendron
lineatum. J. Chem. Ecol. 23:2479-2491
Botterweg, P.F. (1982) Dispersal and flight behaviour of the spruce bark beetle Ips
typographus in relation to sex, size and fat content. Z. Angew. Entomol. 94:466-
489.
Byers, J.A. (1992) Attraction of bark beetles, Tomicus piniperda, Hylurgops
palliatus, and Trypodendron domesticum and other insects to short-chain alcohols
and monoterpenes. J. Chem. Ecol. 18:2385-2402
Byers, J.A. (1993) Avoidance of competition by spruce bark beetles, Ips
typographus and Pityogenes chalcographus. Experientia 49:272-275
Byers, J.A. (1995) Host tree chemistry affecting colonization in bark beetles. In: Cardé,
R.T.,Bell, W.J. (eds.). Chemical Ecology of Insects 2. Chapman and Hall, New York, pp.
154-213
Byers, J.A. (1996) An encounter rate model for bark beetle populations searching at
random for susceptible host trees. Ecol. Model. 91:57-66
Byers, J.A., Wood, D.L. (1980) Interspecific inhibition of the response of the bark
beetles, Dendroctonus brevicomis and Ips paraconfusus, to their
pheromones in the field. J. Chem. Ecol. 6:149-164
Byers, J.A., Wood, D.L., Craig, J., Hendry, L.B. (1984) Attractive and inhibitory
pheromonesproduced in the bark beetle, Dendroctonus brevicomis, during host
colonization: Regulation of inter- and intraspecific competition. J. Chem. Ecol.
10:861-877
Byers, J.A., Birgersson, G., Löfqvist, J., Bergström, G. (1988) Synergistic pheromones
and monoterpenes enable aggregation and host recognition by a bark beetle,
Pityogenes chalcographus. Naturwissenschaften 75:153-155
Byers, J.A., Lanne, B.S., Löfqvist, J. (1989) Host-tree unsuitability recognized by pine
shootbeetles in flight. Experientia 45:489-492
Byers, J.A., Birgersson, G., Löfqvist, J., Appelgren, M., Bergström, G. (1990) Isolation
of pheromone synergists of bark beetle, Pityogenes chalcographus, from complex
insect-plant odors by fractionation and subtractive-combination bioassay. J. Chem.
Ecol. 16:861-876
Byers, J.A., Schlyter, F., Birgersson, G., Francke, W. (1990) E-Myrcenol in Ips
duplicatus: Anaggregation pheromone component new for bark beetles. Experientia
46:1209-1211
Dickens, J.C., Billings, R.F., Payne, T.L. (1991) Green leaf volatiles: a ubiquitous
chemical signal modifies insect pheromone responses. In. Hrdy, I. (ed.). Insect Chemical
Ecology. Acadamia Praha, Prague, Czechoslovakia, pp. 277-280
Dickens, J.C., Billings, R.F., Payne, T.L. (1992) Green leaf volatiles interrupt aggregation
pheromone response in bark beetles infesting southern pines. Experientia 48:523-524
Forsse, E., Solbreck, C. (1985) Migration in the bark beetle Ips typographus L.:
duration, timing and height of flight. Z. Angew. Entomol. 100:47-57
Francke, W., Heemann, V., Gerken, B., Renwick, J.A.A., Vité, J.P. (1977) 2-Ethyl-1-6-
dioxaspiro[4.4]nonane, principal aggregation pheromone of Pityogenes
chalcographus (L.). Naturwissenschaften 64:590-591
Kimmerer, T. W., Kozlowski, T. T. (1982) Ethylene, ethane, acetaldehyde and ethanol
production by plants under stress. Plant Physiol. 69:840-847
Klimetzek, D., Köhler, J., Vité, J.P. (1986) Dosage response to ethanol mediates host
selection by "secondary" bark beetles. Naturwissenschaften 73:270-272
Kohnle, U., Densborn, S., Kölsch, P., Meyer, H., Francke, W. (1992) (E)-7-methyl-
1-6- dioxaspiro[4.5]decane in the chemical communication of European Scolytidae and
Nitidulidae (Coleoptera). J. Appl. Entomol. 114:187-192
König, G., Brunda, M., Puxbaum, H., Hewitt, C. N., Duckham, S.C., Rudolph, J. (1995)
Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions
of selected mid-European agricultural and natural plant species. Atmosph. Environ.
29:861-874
Moeck, H.A. (1970) Ethanol as the primary attractant for the ambrosia beetle,
Trypodendronlineatum (Coleoptera: Scolytidae). Can. Entomol. 113:939-942
Moeck, H.A., Wood, D.L., Lindahl, K.Q.Jr. (1981) Host selection behavior of bark beetles
(Coleoptera: Scolytidae) attacking Pinus ponderosa, with special emphasis on
the western pine beetle, Dendroctonus brevicomis. J. Chem. Ecol. 7:49-83
Pierce, H.D.Jr., De Groot, P., Borden, J.H., Ramaswamy, S., Oehlschlager, A.C. (1995)
Pheromones in red pine cone beetle, Conophthorus resinosae Hopkins, and its
synonym, C. banksianae McPherson (Coleoptera: Scolytidae). J. Chem. Ecol.
21:169-185
Schlyter, F., Birgersson, G., Leufvén, A. (1989) Inhibition of the attraction to the
aggregationpheromone by verbenone and ipsenol: density regulation mechanisms in bark
beetle Ips typographus. J. Chem. Ecol. 15:2263-2277
Schlyter, F., Löfqvist, J., Jakus, R. (1995) Green leaf volatiles and verbenone modify
attractionof European Tomicus, Hylurgops, and Ips bark beetles.
In: Hain, F.P., Salom, S.M., Ravlin, W.F., Payne, T.L., Raffa, K.F. (eds)
Behavior, Population Dynamics, and Control of Forest Insects. p. 29.
Proceedings IUFRO Working Party Conference. Ohio State Univ.
Schroeder, L.M. (1992) Olfactory recognition of nonhosts aspen and birch by conifer
bark beetles Tomicus piniperda and Hylurgops palliatus. J. Chem. Ecol.
18:1583-1593
Schroeder, L.M., Lindelöw, Ä. (1989) Attraction of scolytids and associated beetles by
different absolute amounts and proportions of à-pinene and ethanol. J. Chem. Ecol.
15:807-817
Thorsteinson, A. J. (1960) Host selection in phytophagous insects. Ann. Rev. Entomol.
5, 193- 218
Wilson, I.M., Borden, J.H., Gries, R., Gries, G.J. (1996) Green leaf volatiles as
antiaggregants for the mountain pine beetle, Dendroctonus ponderosae Hopkins
(Coleoptera: Scolytidae). J. Chem. Ecol. 22:1861-1875

 The bark beetles I. typographus (at left) and P. chalcographus are important
enemies of Norway spruce, P. abies, the dominant tree of northern European forests
[Austarå et al. 1984]. These beetles must find a susceptible host tree and then aggregate in
large numbers by attraction to a pheromone in order to overpower the resinous defenses of
the tree [Byers 1995, 1996]. The host finding phase of bark beetles is the most risky period
of the life cycle, with mortality up to 80 percent or more as judged by ratios of emergence to
entrance hole numbers in bark samples [Byers 1996]. Thus, it would be advantageous for bark
beetles to find their host tree as quickly as possible. Natural selection may have caused bark
beetles to evolve several mechanisms for finding their host tree as well as for avoiding
unsuitable host and nonhost species of plants. Host selection has long been hypothesized to
be a complex process comprising both affinity for host chemicals and avoidance of nonhost
chemicals [Thorsteinson 1960].
The bark beetles I. typographus (at left) and P. chalcographus are important
enemies of Norway spruce, P. abies, the dominant tree of northern European forests
[Austarå et al. 1984]. These beetles must find a susceptible host tree and then aggregate in
large numbers by attraction to a pheromone in order to overpower the resinous defenses of
the tree [Byers 1995, 1996]. The host finding phase of bark beetles is the most risky period
of the life cycle, with mortality up to 80 percent or more as judged by ratios of emergence to
entrance hole numbers in bark samples [Byers 1996]. Thus, it would be advantageous for bark
beetles to find their host tree as quickly as possible. Natural selection may have caused bark
beetles to evolve several mechanisms for finding their host tree as well as for avoiding
unsuitable host and nonhost species of plants. Host selection has long been hypothesized to
be a complex process comprising both affinity for host chemicals and avoidance of nonhost
chemicals [Thorsteinson 1960].

