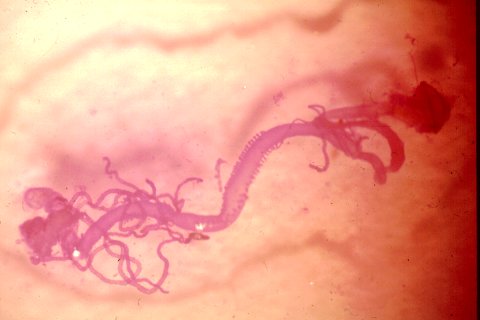
 Mid- and hindgut of Ips paraconfusus bark beetle. The rectum and last abdominal segment are at the
upper right of picture. In the center is the posterior midgut (ventriculus) with the two rows of papillae. Biosynthesis
of ipsenol and ipsdienol my occur in the anterior part of gut according to recent reports.
Mid- and hindgut of Ips paraconfusus bark beetle. The rectum and last abdominal segment are at the
upper right of picture. In the center is the posterior midgut (ventriculus) with the two rows of papillae. Biosynthesis
of ipsenol and ipsdienol my occur in the anterior part of gut according to recent reports.
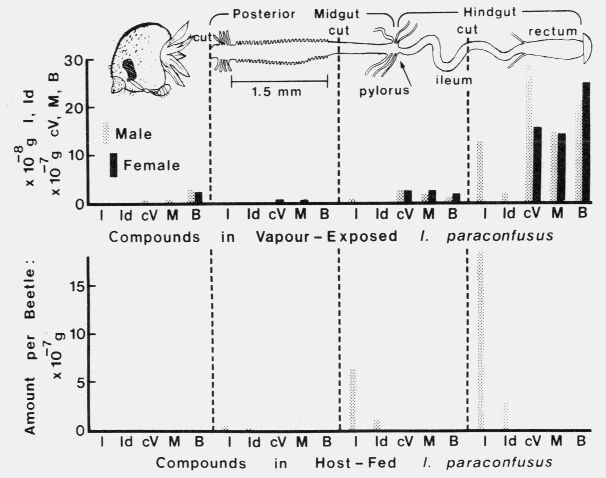
Females, as expected, did not contain ipsenol and ipsdienol (BYERS et al., 1979) while males had a ratio of 5.9 (ipsenol/ipsdienol) in agreement with that in BYERS (1981a) (not 5:9 as misprinted). Both sexes contained relatively large amounts of an unidentified metabolite of myrcene, compound `B' (BYERS and WOOD, 1981), which did not enhance the attraction of beetles to various mixtures of cis-verbenol plus ipsdienol or to ipsenol (Table 2 in BYERS et al., 1979) indicating that compound B cannot substitute for any of the three components. Quantities of the pheromone components and metabolites decreased progressively toward the posterior midgut section. The male and female control groups (whole gut) contained slightly larger quantities of about 130 and 120%, respectively, than that in the gut-sectioned beetles. Host fed females did not contain detectable amounts ( < 0.3 x 10-8 g) of the chemicals shown in Fig. 1. Fed males contained large amounts of ipsenol and ipsdienol again predominately in the posterior hindgut with levels decreasing anteriorly. The fed male control contained approximately 96% as much of these two components as gut-sectioned beetles. The male head and cervical muscles have about twice as much fresh weight (4.9 x 10-4 g) as the mid and hindgut (2.3 x 10-4 g). This indicates that on a per tissue weight basis the heads contain about half that shown in relation to amounts in the hindgut (Fig. 1).
In D. brevicomis the distributions of (-)-alpha-pinene metabolites, trans-verbenol and myrtenol, in the digestive tract were somewhat different than that in I. paraconfusus since about equal amounts of each compound were found in the anterior and posterior sections of the hindgut (Fig. 2).
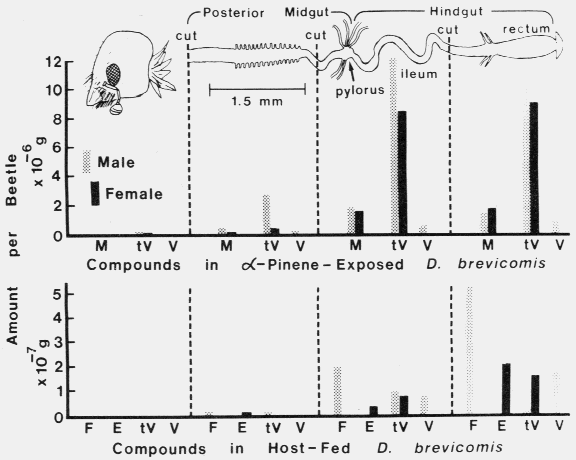
Fig. 2. Distribution of pheromonal compounds and volatiles in various sections of the digestive tract of male and female D. brevicomis exposed to vapour of ( - )-alpha-pinene (18 hr) or allowed to feed together in pine logs (females for 74 hr, males added last 26 hr). Myrtenol = M, trans-verbenol = tV, verbenone = V, frontalin = F and exo-brevicomin = E. The absence of a compound indicates that none was detected by GLC ( < 0.3 x 10-8 g/beetle).
Verbenone was not produced in females, but in males its levels were highest in the posterior hindgut. Very small amounts of trans-verbenol were found in the head and even smaller if based on a fresh weight basis. Control groups of males and females contained about 69 and 72% as much of the compounds as gut-sectioned beetles. Fed males contained increasing amounts of frontalin and verbenone toward the posterior end of the gut. However, trans-verbenol was highest in the anterior hindgut, although a difference has not been shown statistically. Fed females showed the same general distribution pattern for exo-brevicomin and trans-verbenol as that for the male's frontalin, but females did not contain either frontalin or verbenone (Fig. 2). Fed control groups of males and females had about 83 and 74% as much pheromone components as gut-sectioned beetles. The morphology of male and female digestive tracts of D. brevicomis appeared identical with the light microscope ( x 100) as did the guts of male and female I. paraconfusus.
Accumulation of ipsenol, trans-verbenol and myrtenol in the hindgut of I. paraconfusus after exposure of adults to these vapours
In beetles exposed to vapours of ipsenol, trans-verbenol and (-)-myrtenol, large amounts of these compounds were absorbed or accumulated in the mid and hindgut as compared to the head (Table 1).
| Table 1. Accumulation of ipsenol, trans-verbenol and ( - )-myrtenol in hindguts of mature I. paraconfusus during exposure to these vapours for 18 hr | ||||
| Quantity (x 10-8g) per body part | ||||
|---|---|---|---|---|
| Vapour exposure: Body part extracted | Vapour concentration (x 10-8 g/ml) | Ipsenol | trans-verbenol | (-)-myrtenol |
| Ipsenol: | ||||
| Male hindgut | 24.9 | 362.7 | 0* | 0 |
| Male head | 24.9 | 1.0 | 0 | 0 |
| Trans-verbenol: | ||||
| Male hindgut | 6.2 | 0 | 394.2 | 0 |
| Male head | 6.2 | 0 | 1.2 | 0 |
| (-)-Myrtenol: | ||||
| Male hindgut | 4.4 | 0 | 0 | 157.3 |
| Male body, no gut | 4.4 | 0 | 0 | 38.0 |
| Female hindgut | 3.8 | 0 | 0 | 146.4 |
| Female body, no gut | 3.8 | 0 | 0 | 21.6 |
However, some (-)-myrtenol was found in/on degutted males and females exposed to vapours of this compound but less than that contained in the gut.
Pheromones produced in immature and mature bark beetles exposed to plant monoterpene vapours
Monoterpene alcohols were not found in either callow or adult I. paraconfusus which had not been exposed to host-plant vapours (Table 2).
| Table 2. Pheromones produced in callow and mature males and females of I. paraconfusus exposed to vapours of mycene and alpha-pinene or no vapours and the attractive response of females to gut extracts from these vapour-exposed beetles | ||||||
| Quantity (x 10-8g) per gut | ||||||
|---|---|---|---|---|---|---|
| Maturity: sex | Vapour exposure % Responding to gut extracts* (95% B.C.L.) | Ipsenol | cis-verbenol | Ipsdienol | trans-verbenol | |
| Callow malea | No terpenes | 7 (0-22) | 0b | 0 | 0 | 0 |
| Callow femalea | No terpenes | 7 (0-12) | 0 | 0 | 0 | 0 |
| Callow malea | Myrcene/alpha-pinenec | 7 (0-22) | 0 | 120.6 | -d | 162.5 |
| Callow malee | Myrcene/alpha-pinenec | 2 (0-9) | 0 | 48.2 | - | 77.9 |
| Callow femalea | Myrcene/alpha-pinenec | 5 (1-14) | 0 | 29.0 | - | 43.4 |
| Callow malef | Myrceneg | 3 (0-12) | 0 | 0 | 0 | 0 |
| Callow femalef | Myrceneg | 4 (0-12) | 0 | 0 | 0 | 0 |
| - | ||||||
| Mature malee | No terpenes | 3 (1-12) | 0 | 0 | 0 | 0 |
| Mature femalee | No terpenes | 2 (0-9) | 0 | 0 | 0 | 0 |
| Mature malea | Myrcene/alpha-pinenec | 43 (25-62) | 16.0 | 217.5 | - | 280.4 |
| Mature malee | Myrcene/alpha-pinenec | 48 (35-61) | 7.6h | 182.0 | - | 253.7 |
| Mature femalea | Myrcene/alpha-pinenec | 8 (2-18) | 0 | 111.9 | - | 190.7 |
| Mature malef | Myrceneg | 58 (45-70) | 35.7h | 0 | 7.2h | 0 |
| Mature femalef | Myrceneg | 4 (0-12) | 0 | 0 | 0 | 0 |
a27 February 1976, for each row 14-20 insects extracted x 1 replicate; and N = 30 tested for response.
bAll zeros indicate that no compound was detected by GLC (< 0.3 x 10-8 g/gut).
cBeetles exposed for 18 hr in a jar to vapours in equilibrium with 150 µl myrcene and 100 µl (- ) and 100 µl (+ ) alpha-pinene.
dIpsdienol quantities could not be determined as it elutes with trans-verbenol (from alpha-pinene exposure), however, ipsdienol has been shown consistently to be about 1/6 that of ipsenol (BYERS, 1981a).
e3 March 1976, for each row 10-12 insects extracted x 2 replicates; and N = 60 tested for response.
f29 March 1976, for each row 20 insects extracted x 2 replicates; and N = 60 tested for response.
gBeetles exposed for 18 hr in a jar to vapours of GLC-purified myrcene (24.8 x 10-7 g myrcene/ml, S.E.M. = 1.8 x 10-7 g/ml, N = 8).
hlndicates that the amount of compound was significantly greater than other values in the same column on each date (alpha = 0.05, t-test).
Both callow males and females produced cis- and trans-verbenol during exposure to vapours of alpha-pinene enantiomers, although somewhat less than mature adults (Table 2). Neither callow males or females nor mature females could synthesize ipsenol and ipsdienol from myrcene while these pheromone components were made in mature males. The walking response of females was significantly higher only to those gut extraets shown by GLC to contain ipsenol and ipsdienol and in some also cis-verbenol (Table 2).
Pupae and callow adults of both sexes of D. brevicomis did not contain detectable quantities of pheromonal compounds when they were not exposed to host-plant vapours (Table 3).
| Table 3. Pheromone components produced in pupae, callow and mature males and females of D. brevicomis exposed to vapours of (-)-alpha-pinene (7.8 ± 0.8 x 10-8 g/ml, ± S.E.M., N = 12) for 18 hr (23 April - 3 May 1980). | ||||||
| Quantity (x 10-8g) per gut ± S.E.M. | ||||||
|---|---|---|---|---|---|---|
| Maturity: sex | (Number) x Groups exposed to: | F | E | tV | V | M |
| (10-15) x 1 | ||||||
| Pupae male | No terpenes | 0* | 0 | 0 | 0 | 0 |
| Pupae female | No terpenes | 0 | 0 | 0 | 0 | 0 |
| Callow male | No terpenes | 0 | 0 | 0 | 0 | 0 |
| Callow female | No terpenes | 0 | 0 | 0 | 0 | 0 |
| (20) x 6 | ||||||
| Mature male | No terpenes | 24 | 0 | 30 ± 5a | 279 ± 36a | 8 ± 3 |
| Mature female | No terpenes | 0 | 0 | 66 ± 13a | 0 | 6 ± 1 |
| (10-15) x 2 | ||||||
| Pupae male + female | alpha-pinene vapour | 0 | 0 | 4 ± 2 | 0 | 1 ± 0.5 |
| Callow male | alpha-pinene vapour | 0 | 0 | 226 ± 30 | 0 | 34 ± 8 |
| Callow female | alpha-pinene vapour | 0 | 0 | 148 ± 51 | 0 | 23 ± 10 |
| (20) x 6 | ||||||
| Mature male | alpha-pinene vapour | -c | - | 2181 ± 206b | 229 ± 51a | 286 ± 4a |
| Mature female | alpha-pinene vapour | - | - | 2088 ± 289a | 0 | 376 ± 55a |
*Zeros indicate that no compound was detected by GLC (< 0.3 x 10-8 g/gut).
aIndicates that the amounts were significantly different from the corresponding treatment of the callow sex (t-test, alpha = 0.05).
bQuantities not determined.
Mature adults of both sexes contained trans-verbenol and myrtenol, but frontalin and verbenone were found only in males, and no detectable levels of exo-brevicomin were found in females. Both callow males and females produced trans-verbenol and myrtenol during exposure to high concentrations of (-)-alpha-pinene while pupae had only traces of these compounds under the same conditions. Frontalin, exo-brevicomin and verbenone were not found in these immature beetles exposed to (-)-alpha-pinene vapour. Exposure of mature males and females to ( - )-alpha-pinene vapours resulted in large quantities of trans-verbenol and myrtenol, but did not appear to increase the amount of verbenone already present in emerged males and no verbenone was detected in females (Table 3).
Some of the first studies which indicated that the digestive tract of bark beetles contain pheromones were reported by WOOD and BUSHING (1963) and VITÉ et al. (1963) who found pheromone activity in faecal pellets. Later reports showed pheromone activity associated with hindgut extracts (PITMAN et al., 1965; ZETHNER-MOLLER and RUDINSKY, 1967) and Volatile compounds suspected of being pheromonal were found in hindguts by RENWICK et al. ( 1966), PITMAN et al. (1966) and RENWICK and VITÉ (1972). Identified pheromones have been isolated from the hindguts of many bark beetles by PITMAN et al. (1969), VITÉ et al. (1972), HUGHES (1973, 1975), RENWICK et al. (1976), COSTER and VITÉ (1972), BORDEN (1974), BAKKE et al. (1977), and BYERS and WOOD (1980). However, bark beetle pheromones have been found in two cases elsewhere than the digestive tract. HUGHES (1973) identified trans-verbenol from haemolymph withdrawn with a syringe from D. valens and D. ponderosae. He proposed that this indicated its synthesis occurred in the haemocoel and was then transported via the Malpighian tubules to the hindgut to be concentrated by reabsorption of water. However, the possibility that the trans-verbenol had diffused from the hindgut or had been reabsorped by the cryptonephridial Malpighian tubules cannot be excluded. In the other case, GORE et al. (1977) believe that alpha-multistriatin is located in an accessory gland near the anus of Scolytus multistriatus. Another pheromone component, 4-methyl-3-heptanol, was found in the abdomen but neither compound was detected in the hindgut.
In the present study when I. paraconfusus was exposed to plant monoterpene vapours, there were no apparent differences within the gut in the distributions of the metabolites of myrcene, ipsenol and ipsdienol (ipsdienol system), and in the distributions of the metabolites of alpha-pinene, cis-verbenol and myrtenol (cis-verbenol system). However, as shown previously (BYERS et al., 1979) females do not have the ipsdienol system. On the other hand, feeding on the host caused production of large amounts of ipsenol and ipsdienol while cis-verbenol could not be detected. Thus feeding appears to increase the synthetic rate of the ipsdienol system (HUGHES and RENWICK, 1977a) and/or increases the rate of myrcene introduction over vapour exposure alone (BYERS, 1981a). Feeding also appears to inhibit the synthetic rate of the cis-verbenol system or does not provide suffcient alpha-pinene compared to vapour exposure (BYERS,1981a).
It would seem to be more economical for bark beetles to have the biosynthetic sites of pheromones near their release point, the anus, such as in the rectum, so that losses by diffusion into the body would be minimized. Also, it would be more efficient to have the sites of biosynthesis in or near the digestive tract and accessible to host precursors difiusing from the food material. If the insect manufactures pheromone in certain cells or glands in the haemocoel then additional mechanisms are needed to concentrate and excrete the volatiles to the outside via the anus. Furthermore, symbiotic bacteria which have been implicated in the cis-verbenol system (Bacillus cereus, BRAND et aI., 1975) and ipsdienol system (BYERS and WOOD, 1981) in I. paraconfusus would most likely reside in the gut (as most other bacteria do) rather than in the haemocoel.
While it may be reasonable to suspect that the site of pheromone biosynthesis corresponds to the site of accumulation in the hindgut, there is some evidence that synthesis of cis-verbenol may occur in the haemocoel or anterior portion of the gut. The synthesis of cis- and trans-verbenol (from alpha-pinene) and compound B (from myrcene) may not occur in the gut since they were not found in host-fed males while large amounts of ipsenol and ipsdienol were. The quantities of alpha-pinene and myrcene in pine tissue are comparable (BYERS,1981a) so adequate precursors for both the cis-verbenol and ipsdienol systems should have been available in the gut during feeding. However, another explanation which might account for the relative differences between fed males and vapour-exposed males is that synthesis of ipsenol and ipsdienol may reside in the gut epithelium, so that they accumulate, while synthesis of cis-verbenol and other metabolites occur in the gut lumen, and are thus rapidly purged during feeding. This hypothesis would predict large amounts of cis-verbenol relative to ipsenol/ipsdienol in the faecal pellets. However, SILVERSTEIN et al. (1967) found a ratio of 1:267 of cis-verbenol : ipsenol in the frass (boring particles and faecal pellets) which is in agreement with the ratio found in guts of fed males (undetectable cis-verbenol) but opposite to the ratio of 10-20:1 in vapour-exposed males found here and in BYERS (1981a). Furthermore, the cis-verbenol system was not seemingly affected by streptomycin while the ipsdienol system was inhibited (BYERS and WOOD,1981), and this antibiotic, a trisaccharide, is not known to transverse the gut epithelium except in trace amounts, at least in mammals (FRANKLIN and SNOW, 1971). Therefore, this evidence suggests that the ipsdienol system is located in a different place than the cis-verbenol system and that the ipsdienol system is probably in the gut lumen or epithelial cells.
There were no apparent differences in gut morphology between the sexes of each bark beetle species. However, PITMAN et al. (1965) reported that the Malpighian tubules of male I. paraconfusus lengthen about 9 mm as they feed and an earlier conclusion (PITMAN and VITÉ, 1963) that hindgut epithelium of males is thicker than females was retracted. If morphological differences are found between the sexes this may not necessarily be associated with sex-specific pheromone biosynthesis.
In D. brevicomis the distribution of pheromonal compounds in the gut of vapour-exposed beetles was similar to I. paraconfusus. However, it appears that the anterior hindgut of D. brevicomis contained higher amounts relative to the posterior hindgut than in I. paraconfusus. The distributions of compounds in feeding beetles of both species were even more similar. These similarities may either mean that no matter where the pheromones are synthesized they eventually accumulate in the hindgut or that these bark beetles synthesize the compounds in the same general area, the hindgut. An attempt to test these competing hypotheses was done by exposing I. paraconfusus to vapours of certain monoterpene alcohols. If these compounds then had been evenly distributed throughout the insect this would have indicated that the excretory system was not able to concentrate the compounds in the hindgut, and thus it would support the idea that the biosynthetic sites are in the hindgut. However, since the monoterpene alcohols were found in the hindgut, one cannot determine whether this is the result of enzyme/cofactor affinity for the products (monoterpene alcohols) in the hindgut or the result of normal excretory processes. The isolation of various tissues from the beetle and their exposure to pheromone precursors in vitro may provide an answer.
The trans-verbenol/myrtenol production from alpha-pinene in callow D. brevicomis was only about 10% that of mature adults indicating an effect of maturation. However, no pheromones or volatiles were detected in the gut of callow sexes dissected from the bark. Significantly, callow females and males as well as mature females did not make verbenone when exposed to (-)-alpha-pinene while mature males contained verbenone, but the amounts were not apparently different from unexposed males. It is surprising that alpha-pinene apparently was not converted to verbenone, at least at this time, as verbenone is structurally related to trans-verbenol (keto vs alcohol at C4) and no other major host monoterpenes are as closely related as alpha-pinene is to verbenone. The ( + ) enantiomer of alpha-pinene also appears not to be converted to verbenone (BYERS,1983).
HUGHES (1975) reported that D. frontalis males exposed to alpha-pinene contained about twice as much verbenone as unexposed males (GLC peak height of 80.7 vs 44.3 cm). However, in another group of adults just emerging the diflerence apparently was not significant (80.7 vs 73.1 cm). HUGHES (1975) further showed pupae of D. frontalis and D. terebrans did not contain significantly more trans-verbenol after exposure to alpha-pinene, but that adults reared from these pupae contained several times more trans-verbenol than adults reared from unexposed pupae. HUGHES (1975) suggested that pupae may conjugate some form of alpha-pinene with an unknown compound(s) and this conjugate would be later metabolized by the young adult to yield trans-verbenol and verbenone. This hypothesis may explain some of the results of the present study and BYERS (1983) in which trans-verbenol and verbenone quantities increased in adult D. brevicomis held at room temperature for 18 hr in the absence of alpha-pinene vapours. It is also possible that alpha-pinene is not detected in sufficient amounts in the hindgut to account for the production of pheromone components because it is stored in some other part of the body or it is diflused throughout. In this latter hypothesis, pupal exposure may enhance trans-verbenol and verbenone synthesis in adults of Dendroctonus by induction of synthetic enzymes and/or by increasing the precursor reservoir. The changes in pheromone production during maturation are probably further atlected by feeding, as female D. brevicomis at this time produce exo-brevicomin (Fig. 2; HUGHES, 1973) and juvenile hormone application alone caused an even higher production of this component (HUGHES and RENWICK, 1977b).
The present study supports the statement of HUGHES and RENWICK (1977a) that neither callow male nor female I. paraconfusus have a functioning ipsdienol system. Callow adults of both sexes did not have pheromone even though food material was found in almost all beetles. The cis-verbenol system was found to operate in both callow sexes, apparently at a slightly reduced rate compared to mature adults. This could mean that the same oxidative enzymes of the beetle (detoxification?, HUGHES, 1973) or bacteria are present in immature and mature adults. Again, juvenile hormone may be involved with pheromone synthesis (HUGHES and RENWICK, 1977a) by regulating the enzyme system of the beetle or bacteria. Other possibilities are that symbiotic bacteria may be eaten before emergence and/or allowed to flourish during development or feeding. The systems for biosynthesis of pheromones in D. brevicomis and I. paraconfusus appear to be complex and may be affected by sex, developmental stage, bacteria, antibiotic, juvenile hormone, host plant compounds, feeding, mating and ageing (HUGHES, 1973, 1975; BRAND et al., 1975; RENWICK et al., 1976; HUGHES and RENWICK, 1977a,b; BYERS et al., 1979; BYERS and WOOD, 1981; BYERS, 1981a, b, 1982, 1983).
Acknowledgements-This paper was derived in part from my Ph.D. thesis, and I thank D. L. WOOD, Department of Entomological Sciences, University of California at Berkeley for his support, advice and use of the research facilities. I am also grateful to P. SVIHRA and C. S. KOEHLER, Cooperative Extension, University of California at Berkeley, for financial support. P. E. TILDEN, US Forest Service, Oakhurst CA, often helped with collections of brood wood. C. LÖFSTEDT and F. SCHLYTER, Department of Animal Ecology, University of Lund, Sweden, reviewed the manuscript.
BAKKE A., FROYEN P. and SKATTEBOL L. (1977) Field response to a new pheromonal compound isolated from Ips typographus. Naturwissenschaften 64, 98.
BEDARD W. D., TILDEN P. E., LINDAHL K. Q. Jr., WOOD D. L. and RAUCH P. A. (1980) Effects of verbenone and trans-verbenol on the response of Dendroctonus brevicomis to natural and synthetic attractant in the field. J. Chem. Ecol. 6, 997-1013.
BORDEN J. H. (1967) Factors influencing the response of Ips confusus (Coleoptera: Scolytidae) to male attractant. Can. Ent. 99,1164-1193.
BORDEN J. H. (1974) Pheromone mask produced by male Trypodendron Iineatum (Coleoptera: Scolytidae). Can. J. Zool. 52, 533-536.
BRAND J. M., BRACKE J. W., MARKOVETZ A. J., WOOD D. L. and BROWNE L. E. (1975) Production of verbenol pheromone by a bacterium isolated from bark beetles. Nature, Lond. 254,136-137.
BYERS J. A. (1981a) Pheromone biosynthesis in the bark beetle, Ips paraconfusus, during feeding or exposure to vapours of host plant precursors. Insect Biochem. 11, 563-569.
BYERS J. A. (1981b) Eflect of mating on terminating aggregation during host colonization in the bark beetle, Ips paraconfusus. J. Chem. Ecol. 7, 1135-1147.
BYERS J. A. (1982) Male-specific conversion of the host plant compound, myrcene, to the pheromone, (+) ipsdienol, in the bark beetle, Dendroctonus brevicomis. J. Chem. Ecol. 8, 363-371.
BYERS J. A. ( 1983) Insect conversion of a plant compound to enantiomers of an intrasexual inhibitor of pheromone response. Science (submitted). BYERS J. A. and WOOD D. L. (1980) Interspecific inhibition of the response of the bark beetles Dendroctonus brevicomis and Ips paraconfusus to their pheromones in the field. J. Chem. Ecol. 6, 149-164.
BYERS J. A. and WOOD D. L. (1981) Antibiotic-induced inhibition of pheromone synthesis in a bark beetle. Science 213, 763-764.
BYERS J. A., WOOD D. L., BROWNE L. E., FLSH R. H., PIATEK B. and HENDRY L. B. (1979) ReIationship between a host plant compound, myrcene and pheromone production in the bark beetle Ips paraconfusus. J. Insect Physiol. 25, 477-482.
COSTER J. E. and VITÉ J. A. (1972) Effects of feeding and mating on pheromone release in the southern pine beetle. Ann. ent. Soc. Am. 65, 263-266.
FRANKLIN T. J. and SNOW G. A. (1971) Biochemistry of AntimicrobiaI Action. Academic Press, New York.
GORE W. E., PEARCE G. T., LANIER G. N., SIMEONE J. B., SILVERSTEIN R. M., PEACOCK J. W. and CUTHBERT R. A. (1977) Aggregation attractant of the European elm bark beetle, Scolytus multistriatus: production of individual components and related aggregation behavior. J. Chem. Ecol. 3, 429-446.
HUGHES P. R. (1973) Dendroctonus: production of pheromones and related compounds in response to host monoterpenes. Z. angew. Ent. 73, 294-312.
HUGHES P. R. (1974) Myrcene: a precursor of pheromones in Ips beetles. J. Insect Physiol. 20,1271-1275.
HUGHES P. R. (1975) Pheromones of Dendroctonus: origin of alpha-pinene oxidation products present in emergent adults. J. Insect Physiol. 21, 687-691.
HUGHES P. R. and RENWICK J. A. A. (1977a) Neural and hormonal control of pheromone biosynthesis in the bark beetle Ips paraconfusus. Physiol. Ent. 2, 117-123.
HUGHES P. R. and RENWICK J. A. A. (1977b) Hormonal and host factors stimulating pheromone synthesis in female western pine beetles, Dendroctonus brevicomis. Physiol. Ent. 2, 289-292.
PITMAN G. B. and VITÉ J. P. (1963) Studies on the pheromone of Ips confusus (Lec.). I. Secondary sexual dimorphism in the hindgut epithelium. Contr. Boyce Thompson Inst. Pl. Res. 22, 221-226.
PITMAN G. B., KLIEFOTH R. A. and VITÉ J. P. (1965) Studies of the pheromone of Ips confusus (LeConte). II. Further observations on the site of production. Contr. Boyce Thompson Inst. Pl Res. 23,13-17.
PITMAN G. B., RENWICK J. A. A. and VITÉ J. P. (1966) Studies on the pheromone of Ips confusus (LeConte). IV. Isolation of the attractive substance by gas-liquid chromatography. Contr. Boyce Thompson Inst. PI. Res. 23, 243-250.
PITMAN G. B., VITÉ J. P., KINZER G. W. aIld FENTIMAN A. F., JR. (1969) Specificity of population-aggregating pheromones in Dendroctonus. J. Insect Physiol. 15, 363-366.
RENWICK J. A. A. (1967) Identification of two oxygenated terpenes from the bark beetles Dendroctonus frontalis and Dendroctonus brevicomis. Contr. Boyce Thompson Inst. Pl. Res. 23, 355-360. RENWICK J. A. A. and VITÉ J. P. (1970) Systems of chemical communication in Dendroctonus. Contr. Boyce Thompson Inst. Pl. Res. 24, 283-292.
RENWICK J. A. A. and VITÉ J. P. (1972) Pheromones and host volatiles that govern aggregation of the six-spined engraver beetle, Ips calligraphus. J. Insect Physiol. 18, 1215-1219.
RENwICK J. A. A., HUGHES P. R. and KRULL I. S. (1976) Selective production of cis and trans-verbenol from ( - ) and ( + ) alpha-pinene by a bark beetle. Science 191, 199-201.
RENWICK J. A. A., PITMAN G. B. and VITÉ J. P. (1966) Detection of a volatile compound in hindguts of male Ips confusus (LeConte) (Coleoptera: Scolytidae). Naturwissenschaften 53, 83-84.
SILVERSTEIN R. M., RODIN J. O. and WOOD D. L. (1966) Sex attractants in frass produced by male Ips confusus in ponderosa pine. Science 154, 509-510.
SILVERSTEIN R. M., BROWNLEE R. G., BELLAS T. E., WOOD D. L. and BROWNE L. E. (1968) Brevicomin: principal sex attractant in the frass of the female western pine beetle. Science 159, 889-891.
VITÉ J. P. and RENWICK J. A. A. (1970) Differential diagnosis and isolation of population attractants. Contr. Boyce Thompson Inst. Pl. Res. 24, 323-328.
VITÉ J. P., BAKKE A. and RENWICK J. A. A. (1972) Pheromones in Ips: Occurrence and production. Can. Ent. 104, 1967-1975.
VITÉ J. P., GARA R. I. and KLIEFOTH R. A. (1963) Collection and bioassay of a volatile fraction attractive to Ips confusus (LeC.) (Coleoptera: Scolytidae). Contr. Boyce Thompson Inst. PI. Res. 22, 39-50.
WOOD D. L. (1970) Pheromones of bark beetles. In Control of Insect Behavior by Naturat Products (edited by WOOD D. L., SILVERSTEIN R. M. and NAKAJIMA M.), pp. 301-306.
WOOD D. L. (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. A. Rev. Ent. 27, 411-446.
WOOD D. L. and BUSHING R. W. (1963) The olfactory response of Ips confusus (LeConte) (Coleoptera: Scolytidae) to the secondary attraction in the laboratory. Can. Ent. 95,1066-1078.
WOOD D. L., BROWNE L. E., SILVERSTEIN R. M. and RODIN J. O. (1966) Sex pheromones of bark beetles-I. Mass production, bioassay, source, and isolation of the sex pheromone of Ips confusus (LeC.). J. Insect Physiol. 12, 523-536.
WOOD D. L., STARK R. W., SILVERSTEIN R. M. and RODIN J. O. (1967) Unique synergistic efiects produced by the principal sex attractant compounds of Ips confusus (LeConte) (Coleoptera: Scolytidae). Nature, Lond. 215, 206.
WOOD D. L., BROWNE L. E., BEDARD W. D., TILDEN P. E., SILVERSTEIN R. M. and RODIN J. O. ( 1968) Response of Ips confusus to synthetic sex pheromones in nature. Science 159, 1373-1374.
ZETHNER-MOLLER O. and RUDINSKY J. A. (1967) Studies on the site of sex pheromone production in Dendroctonus pseudotsugae (Coleoptera:Scolytidae). Ann. ent. Soc. Am. 60, 572-562.
 Mature (emerged) and callow (immature, yellow, and unemerged) adults of Ips paraconfusus
Mature (emerged) and callow (immature, yellow, and unemerged) adults of Ips paraconfusus
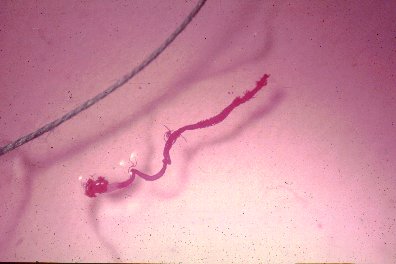 Mid- and hindgut of Ips paraconfusus stained purple compared to a blue sewing thread.
Mid- and hindgut of Ips paraconfusus stained purple compared to a blue sewing thread.