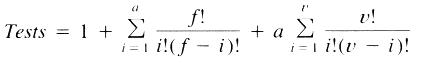 (1)
(1) Attraction of bark beetles (Ips paraconfusus) to aggregation pheromone released upwind in
a laboratory olfactometer.
Attraction of bark beetles (Ips paraconfusus) to aggregation pheromone released upwind in
a laboratory olfactometer.

 Bark beetles such as Ips paraconfusus detect aggregation pheromone components with their
antennae. The laboratory bioassay (or olfactometer) for isolating semiochemicals of bark beetles allows
the insects to walk about in an arena where a laminar air flow carries volatiles "downwind"
and the beetles may orient toward the source of attractants.
Bark beetles such as Ips paraconfusus detect aggregation pheromone components with their
antennae. The laboratory bioassay (or olfactometer) for isolating semiochemicals of bark beetles allows
the insects to walk about in an arena where a laminar air flow carries volatiles "downwind"
and the beetles may orient toward the source of attractants.
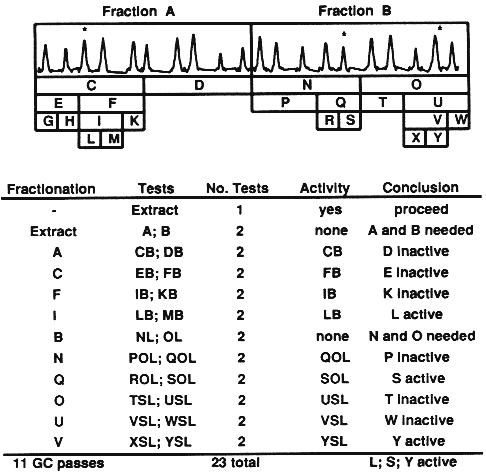
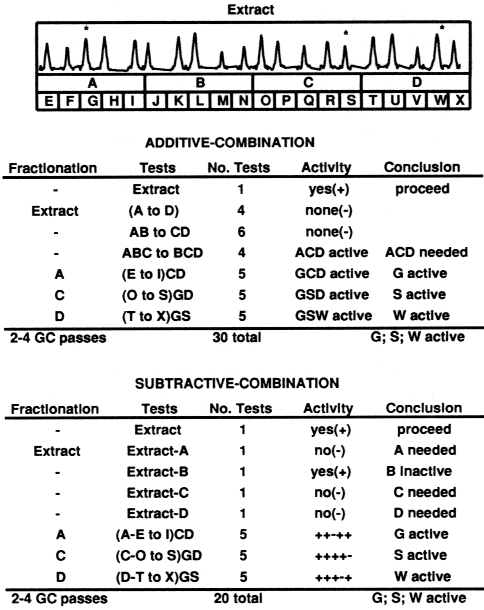
| All combinations of three things selected from five | = | C53 | = | 5! -------------- 3! (5 - 3)! | = | 10 |
|---|
| All permutations of three things selected from five | = | P53 | = | 5! -------------- (5 - 3)! | = | 60 |
|---|
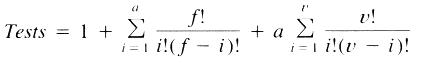 (1)
(1)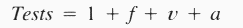 (2)
(2)
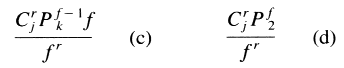
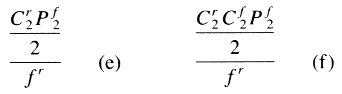
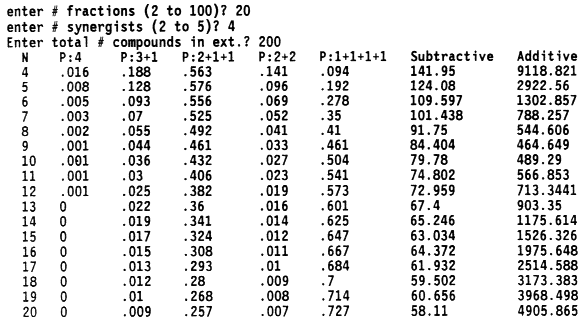
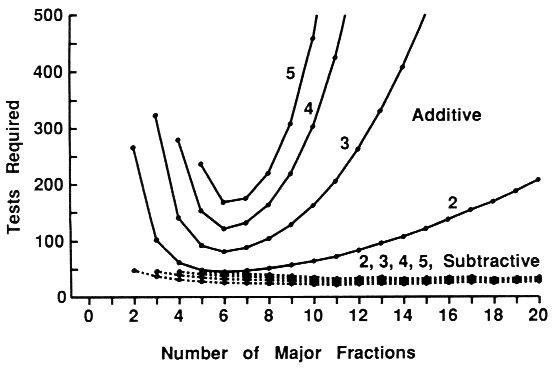
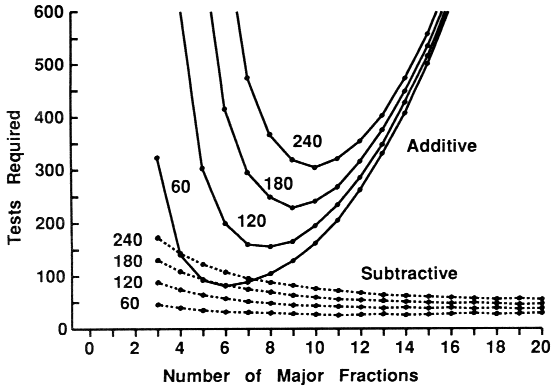
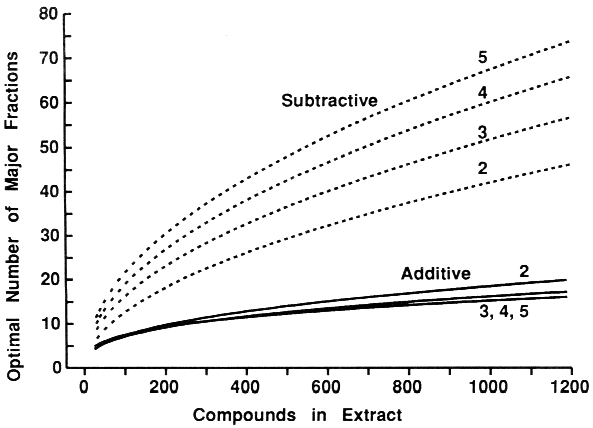
| Table 1. Expected number of bioassay tests required for isolation of two to five synergists in extracts of any number of compounds (n) and with initial fractionation into any number of fractions (f)a | ||||
| Two synergists | ||||
|---|---|---|---|---|
| Additive-combination tests = 1 + p2C1f + p1+1[SUM i=1 to 2]Cif + p2[SUM i=1 to 2]Cin + p1+12C1n | ||||
| Subtractive-combination tests = 1 + f + p2C1n + p1+12C1n | ||||
| Three synergists | ||||
| Additive-combination tests = 1 + p3C1f + p2+1[SUM i=1 to 2]Cif + p1+1+1[SUM i=1 to 3]Cif + p3[SUM i=1 to 3]Cin+ p2+1{ [SUM i=1 to 2]Cin + C1n} + p1+1+13C1n | ||||
| Subtractive-combination tests = 1 + f + p3C1n + p2+12C1n + p1+1+13C1n | ||||
| Four synergists | ||||
| Additive-combination tests = 1 + p4C1f + p3+1[SUM i=1 to 2]Cif + p2+2[SUM i=1 to 2]Cif + p2+1+1[SUM i=1 to 3]Cif + p1+1+1+1[SUM i=1 to 4]Cif + p4[SUM i=1 to 4]Cin + p3+1{ [SUM i=1 to 3]Cin + C1n} + p2+2{ [SUM i=1 to 2]Cin + [SUM i=1 to 2]Cin} + p2+1+1{ [SUM i=1 to 2]Cin + 2C1n} + p1+1+1+14C1n | ||||
| Subtractive-combination tests = 1 + f + p4C1n + p3+12C1n + p2+22C1n + p2+1+13C1n + p1+1+1+14C1n | ||||
| Five synergists | ||||
| Additive-combination tests = 1 + p5C1f + p4+1[SUM i=1 to 2]Cif + p3+2[SUM i=1 to 2]Cif + p3+1+1[SUM i=1 to 3]Cif + p2+2+1[SUM i=1 to 3]Cif + p2+1+1+1[SUM i=1 to 4]Cif + p1+1+1+1+1[SUM i=1 to 5]Cif + p5[SUM i=1 to 5]Cin + p4+1{ [SUM i=1 to 4]Cin + C1n} + p3+2{ [SUM i=1 to 3]Cin + [SUM i=1 to 2]Cin} + p3+1+1{ [SUM i=1 to 3]Cin + 2C1n} + p2+2+1{2[SUM i=1 to 2]Cin + C1n} + p2+1+1+1{ [SUM i=1 to 2]Cin + 3C1n} + p1+1+1+1+15C1n | ||||
| Subtractive-combination tests = 1 + f + p5C1n + p4+12C1n + p3+22C1n + p3+1+13C1n + p2+2+13C1n + p2+1+1+14C1n + p1+1+1+1+15C1n | ||||
|
JOHN A. BYERS Department of Animal Ecology, Lund University, SE-223 62 Lund, Sweden Present address: 
|
|---|