Byers, J.A. 1983. Sex-specific responses to aggregation pheromone:
Regulation of Colonization Density in the Bark Beetle
Ips paraconfusus
Journal of Chemical Ecology 9:129-142.  pdf
pdf
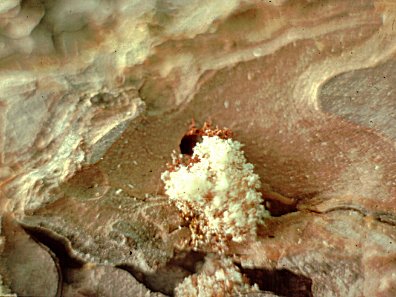 Attack by a male Ips paraconfusus into bark of ponderosa pine showing the "glued" fecal pellets (reddish) around the entrance
hole and the xylem sawdust frass (yellow).
Attack by a male Ips paraconfusus into bark of ponderosa pine showing the "glued" fecal pellets (reddish) around the entrance
hole and the xylem sawdust frass (yellow).
Abstract--
About equal numbers of each sex of flying Ips paraconfusus
Lanier (Coleoptera: Scolytidae) were caught on traps several meters
downwind from a male-infested ponderosa pine log releasing pheromone
while a significantly different ratio of over four times more females than
males were caught at the pheromone source. Females oriented directly to
higher concentrations of colonizing males in a felled tree while males tended
to land on the host in adjacent uncolonized areas. The attraction response of
walking males to a 1: 1 : 1 mixture of the synthetic pheromone components
ipsenol-ipsdienol-cis-verbenol was reduced progressively at higher concentrations while
female response continued to increase. These responses
may function to regulate density of colonization and limit intraspecific
competition.
Key Words-
Coleoptera, Scolytidae, Ips paraconfusus, bark beetle, Pinus ponderosa,
ipsenol, ipsdienol, cis-verbenol, pheromone, attractant, intra-specific competition.
INTRODUCTION
Most bark beetle species studied, including Ips paraconfusus Lanier
(Coleoptera: Scolytidae), use pheromones to attract individuals of both sexes
to a suitable host tree for breeding (Borden, 1977). Each species probably has
evolved behavioral and physiological mechanisms which attenuate aggregation
that would otherwise result in overcolonization and severe competition
for food and space. In the bark beetle, Dendroctonus brevicomis (Renwick
and Vité, 1970; Bedard et al., 1980a), D. pseudotsugae (Rudinsky et al., 1972,
1974), and Trypodendron lineatum (Nijholt, 1973) inhibitory pheromones
may assist in regulating the density of colonization and/or termination of
aggregation on host materials. The reduction or inhibition of attraction
response in these beetles either affects both sexes or may be limited to one,
such as T. lineatum in which effects on females have not been determined. One
of the intraspecific inhibitors of attraction response in D. brevicomis,
verbenone, also decreases response of its competitor, I. paraconfusus, to its
conspecific pheromone (Byers and Wood, 1980). Attractive pheromone
components of certain Ips species may, in addition, function to inhibit
response of competing bark beetle species to their conspecific pheromone
(Birch and Wood, 1975; Birch et al., 1980; Byers and Wood, 1981).
Male I. paraconfusus begin colonizing ponderosa pine, Pinus ponderosa
Laws., by excavating a "nuptial chamber" under the bark cortex primarily in
the phloem layer. Two attractive pheromone components, ipsenol and
ipsdienol (Silverstein et al., 1966), are synthesized only in males from the host
terpene, myrcene (Hughes, 1974; Byers et al., 1979; Hendry et al., 1980). A
third pheromone component, cis-verbenol, is synthesized from another host
compound, alpha-pinene, in both sexes (Renwick et al., 1976; Byers, 1981 a). The
three components together are attractive to both males and females in the field
(Wood et al., 1968). I. paraconfusus males appear to regulate their attack
density since Struble and Hall (1955) reported a maximum of 374 attacks/m2
with the majority of samples ranging from 121 to 186/m2. The density of
females infesting pine is proportional to the density of males which allow entry
of up to five females (usually three) into the nuptial chamber. Subsequently,
others seeking entry are forcefully rejected (Barr, 1969). A recent study has
indicated that the attraction of male I. paraconfusus to logs infested with
males was inhibited by volatiles from an additional log infested with mated
males and females (Byers, 1981b). This study implicated an olfactory
mechanism that may regulate the attack density. The present study reports
differences between the sexes in their upwind flight to a male-infested log and
in their attraction to a concentration series of pheromone components. In
addition, differences between the sexes in landing patterns on a felled tree
under colonization reveal a mechanism of male inhibition by high levels of
pheromone, which appears to regulate colonization density and intraspecific
competition.
METHODS AND MATERIALS
 Experimental Grid of sticky-traps, wind vane, and log of 50 males (right of center in foreground).
Experimental Grid of sticky-traps, wind vane, and log of 50 males (right of center in foreground).
Upwind Flight Response to Pheromone. The pattern of flying beetles of
each sex orienting to pheromone released by 50 males boring in a ponderosa
pine log was determined by intercepting their flight with a grid of sticky-traps
placed downwind from the infested log (September 20-28, 1975). The grid of
traps (Figure 1) was located in a narrow valley in the Sierra National Forest
(Miami Creek drainage at 1200 m elevation, Mariposa County, California).
Observations of a wind-vane placed in various positions in the grid indicated a
consistent wind direction perpendicular to the trap rows throughout the flight
period (time of catch from about 0800 to 2000) probably due to solar heating
at lower elevations forcing air upslope in a laminar flow.
 Sticky trap surrounding screened log with 50 male Ips paraconfusus that served as pheromone
source.
Sticky trap surrounding screened log with 50 male Ips paraconfusus that served as pheromone
source.
The male-infested log was screened and prepared as described earlier
(Byers and Wood, 1980). It was placed inside a tubular sticky-trap (19 cm
diam X 30.5 cm high) made of 6.3-mm mesh screen coated with Stickem
Special® and 1.2 m above the ground (Bedard and Browne, 1969). Each row of
traps had a nylon line perpendicular to the wind direction which suspended 15
flat sticky-trap screens (each 30.5 X 61 cm high) coated with Stickem Special
and spaced at 1.5 m intervals 1.2 m above ground. The sticky-traps were
monitored for beetles two days prior to introducing the infested log and six
days after its removal. The "weighted" mean distances of catch from the
pheromone source for each row and sex were calculated from numbers caught
on traps 3-13 (and their respective distances) in each row.
Walking Response to Synthetic Pheromone Components. Beetles used
for tests in a laboratory olfactometer (Byers et al., 1979, modified from
Browne et al., 1974) were collected from naturally infested pine logs obtained
from the same area described above. Walking beetles of each sex were tested
for their attraction response to a series of increasing concentrations of a 1: 1 : 1
mixture of ipsenol-ipsdienol-cis-verbenol (racemic chemicals) < 97% pure,
Chemical Samples Co.). Concentrations of each compound ranged in decimal
steps from 10-11 to 10-6 g/µl diethyl ether.
Landing on Trunk of Felled Pine during Colonization. Numbers of male
and female I. paraconfusus caught on sticky-traps along the trunk of a felled
pine were analyzed to determine whether behavioral differences between the
sexes existed under conditions of actual colonization. An apparently healthy
ponderosa pine tree (0.36 m diam at 1.5 m height) was felled at 08:00 on
October 1, 1978, and all branches were removed except those above the 16.3 m
height (trap 1).

Felled ponderosa pine with line of sticky-traps for monitoring landing of Ips paraconfusus during
colonization.
Eighteen sticky-trap screens, 21 X 21 cm, were placed
horizontally on the upper side of the felled tree at intervals of 0.76 m beginning
3.6 m from the "top" (0.1 m diam) and extending along the trunk to 3.3 m from
the base. An aggregation of I. paraconfusus near the top of the tree was
induced by baiting with a log that had been infested two days earlier with 50
males. The male-infested log was removed 24 hr later. The portion of the tree
that was under attack, as indicated by boring dust piles, was noted each day.
The slopes of linear regression of male versus female catch on the 18 traps
for successive two-day periods (October 1-8, 1978) were compared to
corresponding 95% lower binomial confidence limits (LBCL) of sex ratios of
catch (Byers and Wood, 1980) with a t-test method (Mendenhall, 1967). The
total male and female catch and the percentage of males in the catch per day
during the mass aggregation period were summarized.
RESULTS
Upwind Flight Response to Pheromone. The distribution of catch of I. paraconfusus
on the grid of traps appeared as a narrowing pattern focusing to
the pheromone source (Figure 1).
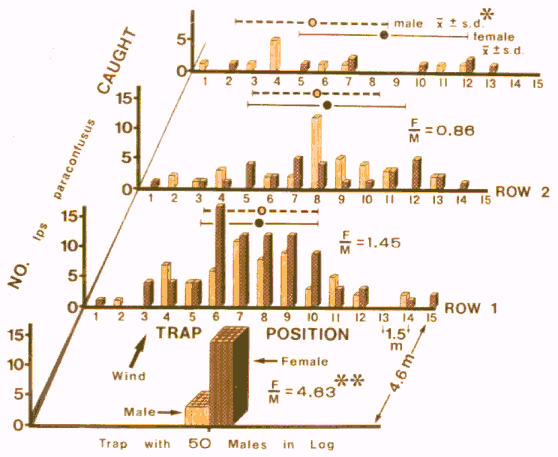
FIG. 1. Distribution of male and female Ips paraconfusus caught on sticky-traps
spaced in a grid downwind from a log infested with 50 males in the Sierra National
Forest, California (September 20-28, 1975). *Mean of distribution of catch ±
standard deviation. **Female/male sex ratio was significantly different from sex
ratios in either row 1 or 2 (P < 0.001, chi-square).
This pattern is to be expected if beetles are
flying upwind toward the source of pheromone. The broad overlaps of the
standard deviations of the sexes caught within each row of traps (Figure 1)
indicated that both sexes. initially directed their flight upwind toward the
pheromone source. The increase in catch on trap rows closer to the source was
probably a function of an increase in the proportion of the cross-sectional area
of the pheromone "odor plume" that was intercepted by trap area. About
equal numbers of male and female beetles appeared to enter the grid area since
catches of each sex on rows 3 and 2 were almost identical. However,
proportionately fewer males than females were attracted upwind to the
source, as the sex ratio caught there was significantly less than on row 1
(Figures 1 and 2), and row 1 was significantly less than rows 2 and 3
(P < 0.05). No beetles were caught after removing the infested log.
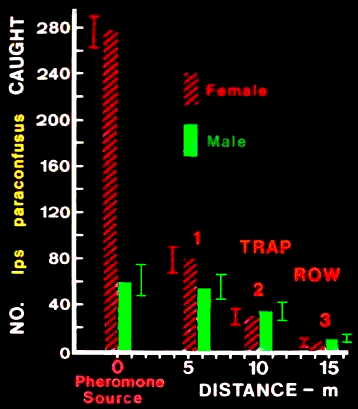
FIG. 2. Total number of male and female Ips paraconfusus caught at the log infested
with 50 males and on sticky-traps (nos. 3-13) in rows of increasing distance downwind
from the pheromone source, Sierra National Forest, California (September 20-28,
1975). The distance from the pheromone source for each row is based on the average
trap distance weighted by the catch. Brackets represent 95% asymmetric binomial
confidence limits.
Walking Response to Synthetic Pheromone Components. Male and
female response was similar at lower release rates (10-11 - 10-9 g/µl) of ipsenol -
cis-verbenol - ipsdienol. However, attraction of males, but not females, was
inhibited at higher release rates (10-8 - 10-6 g/µl, Figure 3).
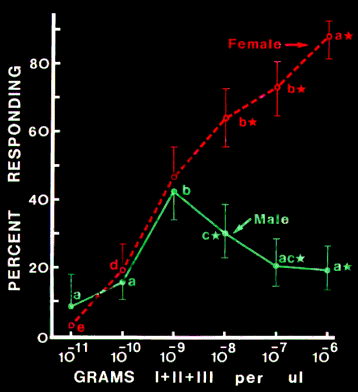
FIG. 3. Response of male and female Ips paraconfusus to a 1:1:1 mixture (separate
weights) of the pheromone components ipsenol (I), cis-verbenol (II), and ipsdienol
(IlI) in the laboratory olfactometer. Means compared within each sex between
concentrations followed by the same letter were not significantly different at alpha = 0.05,
and means compared at the same concentration between sexes followed by star
symbols were significantly different at alpha = 0.01 (chi-square). Brackets represent 95%
binomial confidence limits, N = 90-120.
The actual release
rate (g/min) was estimated to be about 2.2 times the g/µl concentration,
which assumes that the compounds were released in proportion to the volume
reduction of solvent (Byers and Wood, 1981). These results indicated that
response of males increased with an increase in pheromone concentration to a
threshold above which a reduction in attraction occurred. On the other hand,
the attraction of females continued to increase in proportion to the logarithm
of the concentration of pheromone components.
Landing on Trunk of Felled Pine during Colonization. Traps progressively
further from the origin of colonization at the "top" of the felled tree
caught increasingly higher proportions of males on the first, second, and third
days of aggregation (Figure 4).
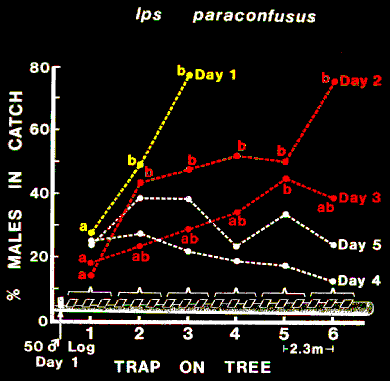
FIG. 4. Trends in the proportion of male Ips paraconfusus caught on sticky-traps
distributed distally from the origin of colonization on the trunk of a felled ponderosa
pine in the Sierra National Forest, California (October 1-5, 1978). An aggregation of I. paraconfusus
near the top of the tree was induced by placing a 50-male-infested log
there for one day only. Proportions within each day followed by the same letter were
not significantly different at alpha = 0.05 (chi-square).
In order to observe these trends in sex ratio it
was necessary to combine the catches of each three successive traps for a total
of six groups. Numerous piles of boring dust were observed only at the "top"
of the tree beginning on the second day. On the third day piles appeared
further along the tree corresponding to the highest catches. By the fourth day,
the entire surface that was covered with traps appeared to be relatively
uniformly attacked (about 110-150 attacks/m2) and no trends in sex ratio
then were observed (Figure 4).
Males responded differently from females to areas of colonization
corresponding to the 18 trap locations on all four two-day periods since the
slopes of the regression lines of male against female catch were significantly
less than the corresponding LBCL (95%) sex ratios of catch on all traps (Table
1).
TABLE I. Comparison of sex ratio of total catch of Ips paraconfusus and
linear regression of male and female catch on 18 sticky-traps along trunk of felled ponderosa pine tree,
Sierra National Forest, California (October 1-8, 1978)
|
| Date | Sex
(males/females) | LBCL (95%)a
sex ratio | Regression slope
(x = female vs. y = male) | Difference between LBCL
and regression slope
(P value)b |
|---|
| Oct. 1-2 | 0.660 | 0.511 | 0.107 | µ 0.001 |
|---|
| Oct. 3-4 | 0.346 | 0.274 | 0.093 | 0.034 |
|---|
| Oct. 5-6 | 0.418 | 0.297 | 0.141 | 0.030 |
|---|
| Oct. 7-8 | 0.300 | 0.185 | -0.078 | 0.027 |
|---|
aLower binomial confidence limit (95%) of thc sex ratio of total catch.
bA P value less than 0.05 indicates the regression slope was significantly less than the LBCL of the
sex ratio.
Similarly, compared to the first and second days of attack, the proportion
of males that landed on the fourth day significantly decreased (each
P < 0.001, chi-square) when female aggregation was the most intense (Figure
5).
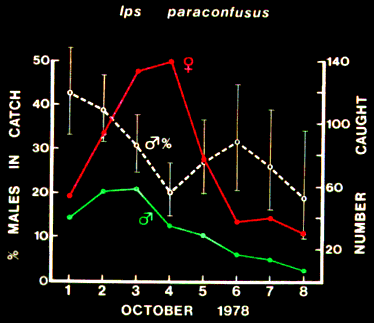
FIG. 5. Relationship between proportion of male Ips paraconfusus caught and the
number of each sex caught daily on sticky-traps on the trunk of a felled ponderosa pine
during colonization in the Sierra National Forest, California (October 1-8, 1978).
DISCUSSION
Previous reports indicated that males were less responsive to pheromone
than females based on their attraction in the laboratory to naturally produced
pheromone (Wood, 1962) or to gut extracts of fed males (Pitman et al., 1965).
The attraction of attacking males to pheromone has been described as an "area
orientation" (higher number of turns per unit distance traveled) compared to
the female's "straight-line orientation" (Wood and Bushing, 1963). It was
further speculated that males would be attracted to host substrates under
colonization but that after landing thigmotactic or gustatory stimulants might
induce attacks on the bark before the source of pheromone, a male entrance
tunnel, was reached. The present study indicates that these earlier observations
were the result of an inhibition of male response to higher concentrations
of ipsenol, cis-verbenol, and ipsdienol. Furthermore, my laboratory bioassays
indicate that the differences in sex ratio of catch in the grid trap experiment
were probably due to inhibition of male response by higher concentrations of
these pheromone components encountered when the male-infested log was
approached. Colonizing males produce the highest amounts of ipsenol and
ipsdienol during the first eight days of an attack (Byers, 1981b), so these levels
of pheromone were available to inhibit the response of males. In the first
experiment, females were not allowed to join males at the pheromone source
so the possibility of release of inhibitors by females, or female-induced release
of inhibitors by males, cannot account for the sex-specific differences in
attraction.
Sex-specific responses were clearly shown in the experiment that
monitored beetles landing on the felled ponderosa pine tree being colonized.
The highest proportion of males in the trap catches occurred farthest from the
origin of attack during the first three days. By the fourth day male attacks had
occurred throughout the trunk, and there were no significant trends in sex
ratio along the trunk, probably because pheromone was being released at all
trap locations. The regression slope of male to female catch was significantly
less than the 95% lower binomial confidence limit of the overall sex ratio of the
catch, a fact which may be explained by inhibition of male response, while
female response increased at those areas of high colonization density and
pheromone concentration. As the colonization of the tree progressed during
the week, the proportion of males in the catch significantly decreased, and it
was lowest at the peak of the aggregation when pheromone release was
presumably the highest (Byers, 1981b). The responding population would
probably arise from several brood locations not necessarily of the same age
that each contributed a sex ratio of approximately 1:1 over the period
(Struble and Hall, 1955). Furthermore, based on the beetle's longevity in the
laboratory, it appears that the absolute sex ratio of the population in nature
would be composed of several days' emergence as well as reemerged parent
adults (Struble and Hall, 1955). Thus, the significant change in the sex ratio of
catch observed during the first four days of colonization is probably a result of
differential response of the sexes to varying pheromone release rates rather
than a significant change in the sex ratio of the available adults.
Many investigators of bark beetle biology have wondered about the
discrepancy between the approximate male-female ratio of emerging beetles
(1:1) to the sex ratio caught on traps with male-infested logs (1:3) or the sex
ratio in nuptial chambers (1:3) (Struble and Hall, 1955; Wood, 1962; Gara,
1963; Cameron and Borden, 1967). One previous hypothesis suggested that I. paraconfusus
males sustain higher levels of mortality than females because of
greater exposure to predation and host tree resin during the initial attack
(Gara, 1963). The higher male mortality might result in absolute numbers of
males of about 1/3 those of females in the responding population. However,
the 1:0.86 male-female sex ratio of beetles believed to be entering the grid
area, and the 1:1.35 male-female ratio landing during the first day on the
felled tree, suggests that differences in mortality between the sexes because of
such differences in predation and death during initial burrowing does not
appear to significantly change the sex ratio of flying beetles from that at
emergence.
In this study, males and females appeared to be equally responsive to low
concentrations of pheromone, but many of the males deviated from the path
taken by females when flying to infested hosts to land on areas adjacent to
those with higher densities of males which are releasing higher amounts of
pheromone. This behavioral mechanism would function to regulate density of
colonization and intraspecific competition for food and space. The sexual
difference in response was shown to occur even before beetles landed, and it is
probable that the effect intensifies after landing. Once males are on the host,
other factors may contribute to density regulation and prevent new attacks in
fully infested substrates. If no new attacks occur, the colonized areas would
become nonattractive since ipsenol and ipsdienol quantities in mated males
decrease to negligible levels after 7-10 days (Byers, 1981b).
In contrast to I. paraconfusus, both sexes of the bark beetle Scolytus mutistriatus
have been shown to respond in about equal numbers to sources
of natural attraction (Peacock et al., 1971). Tilden et al. (1979) reported that
more males than females of the monogamous bark beetle, D. brevicomis, were
trapped close to the source of synthetic pheromones than on surrounding
unbaited traps further away. However, the sex ratio was different only
between the source and two comparisons on outlying traps while six other
comparisons at similar distances were not significantly different. In other
studies, the sex ratio of D. brevicomis was found not to be significantly
different from unity during attraction to a trap releasing synthetic pheromones
(Vité and Pitman, 1969; Wood, 1972; Wood et al., 1976; Byers and
Wood, 1980; Bedard et al., 1980b) or, more importantly, to traps containing
naturally infested host logs (Byers and Wood, 1980). Neither sex of D. brevicomis
appeared to be inhibited by increasing the concentrations of its
synthetic pheromone components in the same laboratory walking bioassay
(Byers and Wood, 1981) that demonstrated inhibition of male I. paraconfusus
in the present study. The responses of the sexes of D. brevicomis to individual
components of its pheromone (exo-brevicomin and frontalin) were shown to
be different (Vité and Pitman, 1969), but this may be an artifact since these
compounds are never released alone during mass colonization, except by the
first female (Byers and Wood, 1980). However, certain ratios of these
pheromone components might function to regulate "close-range" behavior
after landing such as mating and avoidance of competition. Verbenone,
produced by male D. brevicomis (Renwick, 1967), inhibits the response of
both sexes to their attractants in the laboratory (Hughes and Pitman, 1971) or
field (Bedard et al., 1980a) and may function to regulate attack density (Byers
and Wood, 1980) or terminate aggregation (Renwick and Vité, 1970). D. frontalis males
also release verbenone which inhibits male response (females
were not tested; Rudinsky, 1973), and synthetic verbenone released at "higher
concentrations" inhibited both sexes (Vité and Renwick, 1970; Payne et al.,
1978).
The attack density and termination of mass attack in D. pseudotsugae
may be controlled in part by release of 3-methyl-2-cyclohexene-1-one (MCH)
from females when a male arrives and stridulates at the entrance hole
(Rudinsky and Michael, 1972). On the other hand, Pitman and Vité (1974)
found that males contain considerably more MCH than females, and they
believe males are primarily responsible for release of the inhibitor. The
responses to pheromone in both sexes are inhibited (Rudinsky et al., 1974),
but males appear to be more strongly affected (Rudinsky et al., 1972). The
density of D. pseudotsugae attack on a tree under colonization has been
reduced by MCH released at multiple sources, and the distance of nearest
attack from MCH elution points increased with the concentration of MCH
(Furniss et at., 1974). Surprisingly, at the highest concentrations of MCH
release, the attack density increased (Hedden and Pitman, 1978). Nijholt
(1973) reported that males of Trypodendron lineatum released an unidentified
inhibitory pheromone after joining the female. Unfortunately, the possibility
of changes in the sex ratio of the responding beetles after inhibitors were
released was not investigated. However, a recent study has shown that female
T. lineatum decrease their release of the attractive pheromone, lineatin, after
the male arrives (Klimetzek et al., 1981). Klimetzek et al. found evidence of a
male-induced inhibition of response which appeared to operate only at close
range and may "involve a communication system other than olfaction"
(possibly acoustic).
The theory that sex-specific differences in response to intraspecific
semiochemicals can function to regulate density of colonization has not been
suggested elsewhere for Ips species. However, Lanier et al. (1972) found
sex-specific differences in I. pini in which females, but not males, from Idaho
could discriminate between New York and Idaho male-infested logs, while
both sexes of New York beetles could differentiate Idaho and New York
pheromone. These differences appear to be the result of differential response
of the geographical populations to enantiomers of ipsdienol (Lanier et al.,
1980). Mustaparta et al. (1980) showed that both Idaho and New York
females (males not tested) had two receptor cells each specialized for a specific
enantiomer of ipsdienol. Since the receptor systems for ipsdienol of the two
populations are virtually identical, the response differences appear to be
governed by the central nervous system rather than the peripheral receptors
(Lanier et al., 1980). Indicative of the present study, Lanier et al. (1980) found
in the laboratory that the responses of male I. pini from New York or Idaho to
odor from male-infested logs were always less than those for the same type of
female. Electrophysiological studies may reveal whether receptors on the
antenna of a male I. paraconfusus (and I. pini ) adapt at lower concentrations
of pheromone than the receptors of females, or whether the sexual difference
in response is a consequence of the central nervous system.
In all Ips species distributed throughout the northern hemisphere, the
males initiate the attack, are polygamous, and in many species studied, the
males produce various ratios and amounts of ipsenol, cis-verbenol, and
ipsdienol (Vité et al., 1972). Therefore, olfactory mechanisms similar to that
described here may operate in reducing intraspecific competition in other
species of the genus Ips.
Acknowledgements-
I thank D.L. Wood, Department of Entomological Sciences, University of California at Berkeley,
for support and use of his research facilities; I also thank W.D.
Bedard and P.E. Tilden of the USDA Forest Service for use of their research facilities at
Oakhurst, California. I am grateful to R.P. Akers, A.M. Liebhold, D.R. Owen, and D.L. Wood,
Department of Entomological Sciences, University of California at Berkeley, and J.S. Elkinton,
Department of Entomology, University of Massachusetts, for helpful reviews of the manuscript.
The work was supported in part by grants from the Rockefeller Foundation, USDA Forest
Service, and Regional Research Project W-110, SEA/USDA (to D.L.W.)
REFERENCES
BARR, B.A. 1969. Sound production in Scolytidae (Coleoptera) with emphasis on the genus Ips.
Can. Entomol. 101:636-672.
BEDARD, W.D., and BROWNE, L.E. 1969. A delivery-trapping system for evaluating insect
chemical attractants in nature. J. Econ. Entomol. 62:1202-1203.
BEDARD, W.D., TILDEN, P.E, WOOD, D.L., LINDAHL, K.Q., and RAUCH, P.A. 1980a. Effects of
verbenone and trans-verbenol on the response of Dendroctonus brevicomis to natural and
synthetic attractant in the field. J. Chem. Ecol. 6:997-1014.
BEDARD, W.D., WOOD, D.L., TILDEN, P.E., LINDAHL, K.Q. JR., SILVERSTEIN, R.M. and
RODIN, J.O. 1980b. Field response of the western pine beetle and one of its predators to host
and beetle produced compounds. J. Chem. Ecol. 6:625-631.
BIRCH, M.C.,and WOOD, D.L 1975. Mutual inhibition of the attractant pheromone response by
two species of Ips (Coleoptera: Scolytidae). J. Chem. Ecol. 1:101-113.
BIRCH, M.C., LIGHT, D.M., WOOD, D.L, BROWNE, L.E., SILVERSTEIN, R.M., BERGOT, B.J.,
OHLOFF, G., WEST, J.R., and YOUNG, J.C. 1980. Pheromonal attraction and allomonal
interruption of Ips pini in California by the two enantiomers of ipsdienol. J. Chem. Ecol.
6:703-717.
BORDEN, J.H. 1977- Behavioral responses of Coleoptera to pheromones, allomones, and
kairomones, pp.169-198. in H.H. Shorey, and J.J. McKelvy, Jr. (eds.). Chemical Control of
Insect Behavior. John Wiley. New York.
BROWNE, L.E., BIRCH, M.C., and WOOD, D.L. 1974. Novel trapping and delivery systems for
airborne insect pheromones. J. Insert Physiol. 20:183-193.
BYERS, J.A. 1981a. Pheromone biosynthesis in the bark beetle, Ips paraconfusus, during feeding or
exposure to vapours of host plant precursors. Insect Biochem. 11:563-569.
BYERS, J.A. 1981b. Effect of mating on terminating aggregation during host colonization in thc
bark beetle, Ips paraconfusus. J. Chem. Ecol. 7:1135-1147.
BYERS, J.A., and WOOD, D.L. 1980. Interspecific inhibition of the response of the bark beetles,
Dendroctonus brevicomis and Ips paraconfusus, to their pheromones in the field. J. Chem.
Ecol. 6:149-164.
BYERS, J.A., and WOOD, D.L.1981a. Interspecific effects of pheromones on the attraction of the
bark beetles, Dendroctonus brevicomis and Ips paraconfusus in the laboratory. J. Chem.
Ecol. 7:9-18.
BYERS, J.A., WOOD, D.L., BROWNE, L.E., FISH, R.H., PIATEK, B., and HENDRY, L.B. 1979.
Relationship between a host plant compound, myrcene and pheromone production in the
bark beetle Ips paraconfusus. J. Insect Physiol. 25:477-482.
CAMERON, E.A., and BORDEN, J.A. 1967. Emergence patterns of Ips confusus (Coleoptera:
Scolytidae) from ponderosa pine. Can. Entomol. 99:236-244.
FURNISS, M.M., DATERMAN, G.E., KLINE, L.F., and RUDINSKY, J.A. 1974. Effectiveness of the
Douglas-fir beetle antiaggregative pheromone methylcyclohexenone at three concentrations
and spacings around felled host trees. Can. Entomol. 106:381-392.
GARA, R.I. 1963. Studies on the flight behavior of Ips confusus (LeC.) (Coleoptera: Scolytidae) in
response to attractive material. Contrib. Boyce Thompson Inst. 22:51-66.
HEDDEN, R.L., and PITMAN, G.B. 1978. Attack density regulation: a new approach to the use of
pheromones in Douglas-fir beetle population management. J. Econ. Entomol. 71:633-637.
HENDRY, L.B., PIATEK, B., BROWNE, L.E., WOOD, D.L., BYERS, J.A., FISH, R.H., and HICKS,
R.A.1980. In vivo conversion of a labelled host plant chemical to pheromones of the bark
beetle Ips paraconfusus. Nature 284:485.
HUGHES, P.R. 1974. Myrcene: A precursor of pheromones in Ips beetles. J. Insect Physiol.
20:1271-1275.
HUGHES, P.R., and PITMAN, G.B. 197I. A method for observing and recording the flight behavior
of tethered bark beetles in response to chemical messengers. Contrib. Boyce Thompson Inst.
24:329-336.
KLIMETZEK, D., KIESEL, K., and MOHRING, C. 1981. Trypodendron lineatum: reduction of
pheromone response by male beetles. Naturwissenschaften 68:148-150.
LANIER, G.N., BIRCH, M.C., STEWART, R.F., and FURNISS, M.M. 1972. Pheromones of Ips pini
(Coleoptera: Scolytidae): Variation in response among three populations. Can. Entomol.
104:1917-1923.
LANIER, G.N., CLASSON, A., STEWART, T., PISTON, J.J., and SILVERSTEIN, R.M. 1980. Ips pini:
The basis for interpopulational differences in pheromone biology. J. Chem. Ecol. 6:677-687.
MENDENHALL, W. 1967. Introduction to Probability and Statistics. Wadsworth, Belmont,
California.
MUSTAPARTA, H., ANGST, M.E., and LANIER, G.N. 1980. Receptor discrimination of enantiomers
of the aggregation pheromone ipsdienol, in two species of Ips. J. Chem. Ecol. 6:689-701.
NIJHOLT, W. W. 1973. The effect of male Trypodendron lineatum (Coleoptera: Scolytidae) on the
response of field populations to secondary attraction. Can. Entomol. 105:583-590.
PAYNE, T.L., COSTER, J.E., RICHERSON, J.V., EDSON, L.J., and HART, E.R. 1978. Field response of
the southern pine beetle to behavioral chemicals. Environ. Entomol. 7:578-582.
PEACOCK, J.W., LINCOLN, A.C., SIMEONE, J.B., and SILVERSTEIN, R.M. 1971. Attraction of
Scolytus multistriatus (Coleoptera: Scolytidae) to a virgin-female-produced pheromone in
the field. Ann. Entomol. Soc. Am. 64:1143-1149.
PITMAN, G.B., and VITÉ, J.P. 1974. Biosynthesis of methylcyclohexenone by male Douglas-fir
beetle. Environ. Entomol. 3:886-887.
PITMAN, G.B., KLIEFOTH, R.A., and VITÉ, J.P. 1965. Studies on the pheromone of Ips confusus
(LeConte). II. Further observations on the site of production. Contrib. Boyce Thompson
Inst. 23:13-17.
RENWICK, J.A.A. 1967. Identification of two oxygenated terpenes from the bark beetles
Dendroctonus frontalis and Dendroctonus brevicomis. Contrib. Boyce Thompson Inst.
23:355-360.
RENWICK, J.A.A., and VITÉ, J.P. 1970. Systems of chemical communication in Dendroctonus.
Contrib. Boyce Thompson Inst. 24:283-292.
RENWICK, J.A.A., HUGHES, P.R., and KRULL, I.S. 1976. Selective production of cis- and trans-verbenol
from (-) and (+) alpha-pinene by a bark beetle. Science 191:199-201.
RUDINSKY, J.A. 1973. Multiple functions of the southern pine beetle pheromone verbenone.
Environ. Entomol. 2:511-514.
RUDINSKY, J.A., and MICHAEL, R.R. 1972. Sound production in Scolytidae: Chemostimulus of
sonic signal by the Douglas-fir beetle. Science 175:1386-1390.
RUDINSKY, J.A., FURNISS, M.M., KLINE, L.N., and SCHMITZ, R.F. 1972. Attraction and
repression of Dendroctonus pseudotsugae (Coleoptera: Scolytidae) by three synthetic
pheromones in traps in Oregon and Idaho. Can. Entomol. 104:5I5-822.
RUDINSKY, J.A., MORGAN, M.E., LIBBEY, L.M., and PUTMAN, T.B. 1974. Additional components
of the Douglas-fir beetle (Col., Scolytidae) aggregative pheromone and their possible utility
in pest control. Z. Angew. Entomol. 76:65-77.
SILVERSTEIN, R.M., RODIN, J.O., and WOOD, D.L. 1966. Sex attractants in frass produced by
male Ips confusus in ponderosa pine. Science 154:509-510.
STRUBEL, G.R., and HALL, R.C. 1955. The California five-spined engraver its biology and
control. USDA Circular No. 964.
TILDEN, P.E., BEDARD, W.D., WOOD, D.L., LINDAHL, K.Q., and RAUCH, P.A. 1979. Trapping the
western pine beetle at and near a source of synthetic attractive pheromone: Effects of trap
size and position. J. Chem. Ecol. 5:519-531.
VITÉ, J.P., and PITMAN, G.B. 1969. Aggregation behaviour of Dendroctonus brevicomis in
response to synthetic pheromones. J. Insect Physiol. 15:1617-1622.
VITÉ, J.P., BAKKE, A., and RENWICK, J.A.A. 1972. Pheromones in Ips (Coleoptera: Scolytidae):
occurrence and production. Can. Entomol. 104:1967-1975.
WOOD, D.L. 1962. The attraction created by males of a bark beetle Ips confusus (LeConte)
attacking ponderosa pine. Pan-Pac. Entomol. 38:141-145.
WOOD, D.L.1972. Selection and colonization of ponderosa pine by bark beetles, pp. 101-117, in
H.F. van Emden (ed.). Insect/Plant Relationships. Blackwell Sci. Publ., Oxford.
WOOD, D.L., and BUSHING, R.W. 1963. The olfactory response of Ips confusus (LeConte)
(Coleoptera: Scolytidae) to the secondary attraction in the laboratory. Can. Entomol.
95:1066-1078.
WOOD, D.L., BROWNE, L.E., BEDARD, W.D., TILDEN, P.E., SILVERSTEIN, R.M., and RODIN, J.O.
1968. Response of Ips confusus to synthetic sex pheromones in nature. Science
159:1373-1374.
WOOD, D.L., BROWNE, L.E., EWING, B., LINDAHL, K., BEDARD, W.D., TILDEN, P.E., MORI, K.,
PITMAN, G.B., and HUGHES, P.R. 1976. Western pine beetle: specificity among enantiomers of
male and female components of an attractant pheromone. Science 192:896-898.
 Attack by a male Ips paraconfusus into bark of ponderosa pine showing the "glued" fecal pellets (reddish) around the entrance
hole and the xylem sawdust frass (yellow).
Attack by a male Ips paraconfusus into bark of ponderosa pine showing the "glued" fecal pellets (reddish) around the entrance
hole and the xylem sawdust frass (yellow).
 Experimental Grid of sticky-traps, wind vane, and log of 50 males (right of center in foreground).
Experimental Grid of sticky-traps, wind vane, and log of 50 males (right of center in foreground).
 Sticky trap surrounding screened log with 50 male Ips paraconfusus that served as pheromone
source.
Sticky trap surrounding screened log with 50 male Ips paraconfusus that served as pheromone
source.





