Bark beetles (small spots on paper in center) responding to pheromone from feeding male Ips paraconfusus
in the laboratory olfactometer developed at Univ. of Calif. at Berkeley.
Bark beetles (small spots on paper in center) responding to pheromone from feeding male Ips paraconfusus
in the laboratory olfactometer developed at Univ. of Calif. at Berkeley.
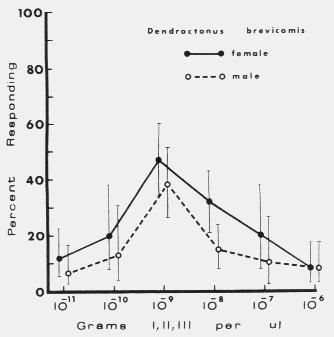
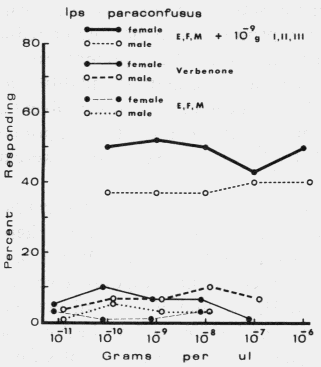
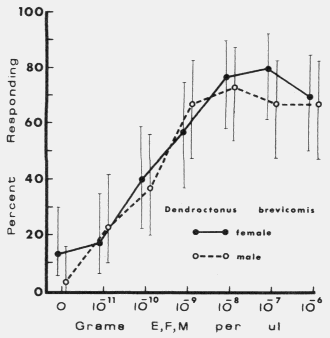
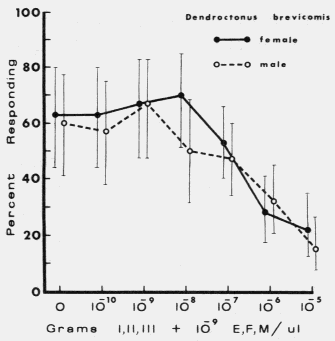
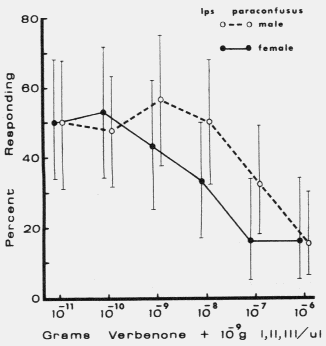
| TABLE 1. Response of male and female Dendroctonus brevicomis to exo brevicomin (E), frontalin (F), and myrcene (M) in various mixtures with ipsenol (I), cis-verbenol (II), and ipsdienol (III) at 0 and 10-10 to 10-5 g each / µl (October 11-12, 1976). Brackets represent 95% binomial confidence limits. | ||||
| Compounds tested [Dose (g/µl)] | Sex | Number tested | Percent responding | Confidence interval (95%0 |
|---|---|---|---|---|
| E, F, M [10-9] | females | 90 | 63 | 52 - 73 |
| E, F, M [10-9] | males | 120 | 68 | 59 - 76 |
| E, F, M [10-9] + I, III [10-6] | females | 30 | 87 | 69 - 97 |
| E, F, M [10-9] + I, III [10-6] | males | 30 | 83 | 64 - 94 |
| E, F, M [10-9] + II [10-6] | females | 60 | 53 | 40 - 68 |
| E, F, M [10-9] + II [10-6] | males | 60 | 60 | 46 - 72 |
| E, F, M [10-9] + I, III [10-6] | females | 90 | 30 | 20 - 41a |
| E, F, M [10-9] + I, II, III [10-6] | males | 90 | 31 | 21 - 42a |