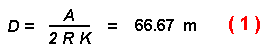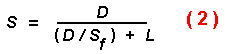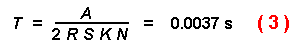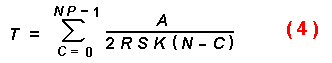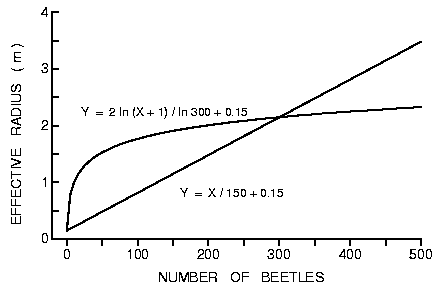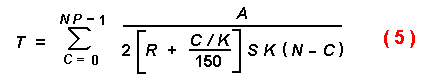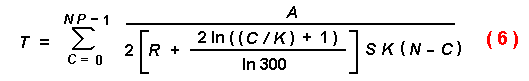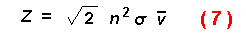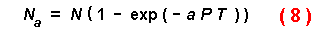Byers, J.A. 1996. An encounter rate model of bark beetle populations
searching at random for susceptible host trees. Ecological Modelling 91:57-66.  pdf
pdf
 Abstract
Abstract
Iterative equations were developed that predict the encounter rate
between a population of moving animals and a population of stationary objects,
where the animals cease to search upon finding an object. The encounter rate
through time depends on the number of searching animals (bark beetles), number
of stationary objects (host trees), average speed of the animals, average
radius of the object, and area of the search arena. The iterative equations
were used in a computer program to vary these parameters with regard to flight
dispersal of the bark beetle Ips typographus (Coleoptera: Scolytidae)
during their search for susceptible host trees of Norway spruce (Picea
abies). Realistic parameters of flight speed, numbers of beetles and
susceptible host trees, tree diameters, density of healthy trees, search area,
and time searching were held constant while certain of these parameters were
varied in computer model runs. In most cases, significant proportions of the
modelled bark beetle population (of which individuals fly forward with a
random component) found the relatively few susceptible host trees suitable for
colonization. Only at very low effective flight speeds (due to longer stays on
trees) or with widely distributed hosts of smaller diameter, did relatively
few beetles find suitable hosts. Once the "pioneer" beetles find susceptible
hosts, release of aggregation pheromone would greatly increase the effective
radius of the host and allow rapid concentration of the population on these
trees. The model suggests that primary attraction to host tree volatiles is
not mandatory for host finding and selection in many species of bark beetle.
Evolution of an olfactory response to host volatiles is more probable in
species with low population densities or widely dispersed host plants, or
both. Also, there would be little selection pressure on a bark beetle species
to evolve aggregation pheromones if they can respond over some meters to plant
volatiles that reveal the location of a susceptible host.
Keywords: Bark beetle; Dispersion; Host-parasite interaction; Search
strategies; Spruce
1. INTRODUCTION
Bark beetles (Coleoptera: Scolytidae) emerge from forest litter or brood
trees and begin a dispersal flight where they seek suitable host trees in
which to reproduce. The dispersal and host-finding phase of their life cycle
is known to be risky, with only 15-25% of the adults successful in finding a
host, boring into the bark, and reproducing in the phloem-cambium layers (as
indicated from ratios of exit holes of brood emergence to entrance holes of
attack, Miller and Keen 1960; Struble and Hall 1955). In a forest of conifer
trees of one or a few species it might seem easy for bark beetles to find a
host. However, healthy trees when injured produce resin that can be toxic and
physically impede and entrap beetles (Smith, 1961; Hodges et al., 1985; Byers,
1995). Usually, only a small fraction of trees in a forest area are unhealthy,
weakened by diseases and insects, or of old age such that they are susceptible
to attack by bark beetles. Unhealthy or injured trees release higher amounts
of volatile chemicals than healthy trees, and these volatiles may serve as
kairomone signals attractive to some species of bark beetles (Graham, 1968;
Moeck, 1970, 1981; Gara et al., 1984; Byers et al., 1985). For example,
ethanol from microbial activity in decaying tissue, and monoterpenes from
wound resin, are well known to be attractive to a number of species (reviews:
Byers, 1992, 1995).
On the other hand, species that attack living trees and use aggregation
pheromones are usually weakly, if at all, attracted to host log odors or
monoterpenes and ethanol (Moeck et al., 1981; Klimetzek et al., 1986; Schlyter
et al., 1987). In some of these "aggressive" species, landing rates have been
observed to be similar on host and non-host trees, suggesting that these
beetles discriminate between hosts and non-hosts only after landing (Berryman
and Ashraf, 1970; Hynum and Berryman, 1980; Moeck et al., 1981; Witanachi and
Morgan, 1981). Thus, a second way bark beetles may find suitable host trees is
by random landing and testing for both the proper host and the tree's
resistance capability (Moeck et al., 1981; Wood, 1982). In this case, the
population of beetles must cooperate so that any individuals that find a
susceptible tree are able to release aggregation pheromone that directs other
searching beetles to join the attack. This cooperation is to the advantage of
the resident beetle as well as those joining since the tree, although weak,
will attempt to resist with resin and might kill one attacking beetle
eventually, but not the combined efforts of many beetles. Because beetles are
difficult to observe flying through the forest, computer models have attempted
to explore dispersal and host-finding behavior (Gries et al., 1989; Byers,
1993).
Gries et al. (1989) computer simulated host selection in Ips
typographus L., a bark beetle using aggregation pheromone when killing
Norway spruce, Picea abies (L.). They concluded this beetle was
unlikely to use random flight and testing of intercepted trees as a mechanism
for host-finding since the beetle would need to fly much farther (at least 60
km) than expected possible in order to find the widely spaced and rare
susceptible host trees. However, their simulation model has deficiencies since
trees were placed in a grid, and beetles took short flights limited to one of
eight possible directions at random between adjacent trees. This flight
pattern is unlikely for bark beetles seeking either host odors or pheromone
that are widely distributed. Beetles would not want to often reverse direction
in a purely random search thereby wasting time on visits to previously
searched areas. Thus, beetles would fly generally forward to cover the most
ground. Furthermore, I have observed bark beetles taking off from a tree to
fly generally straight for tens of meters with some slight
angular component (observations, Byers, 1991, 1995).
A more realistic simulation model would allow beetles to fly forward with
steps where the heading could change gradually at each step in a random way so
that the beetle could assume any position, as illustrated by Skellam (1973).
Also, the trees would be placed at random (or spaced with a degree of
randomness) and could be of any specified size (from trunk size to an
effective attraction radius corresponding to a semiochemical). Thus, the
objective here is to develop a more realistic model of bark beetle host-
finding. The effects on host-finding of Ips typographus are explored in
the model by varying such parameters as the beetle's effective flight speed
and population density as well as the tree's effective radius and density of
susceptible trees.
2. METHODS
A computer simulation model for simulated movement of walking or flying
insects in relation to mate-finding (Byers, 1991) as modified for catch by
pheromone-baited traps (Byers, 1993) was used to simulate finding of
susceptible host trees by bark beetles. In this model, each susceptible tree
(formerly a trap) can catch any number of moving insects. The X- and Y-sides
of the simulation area, number of insects, flight speed, number of trees, tree
radius, and duration of the search period can be varied independently.
Realistic flight of insects in two-dimensions is achieved by using polar
coordinates in which the angle of directional movement is changed randomly at
each step at most equal to the angle of maximum turn (usually ó 30° ),
which can be either right or left (chosen at random) from the previous
direction. The step length (usually about 0.5% of the area's side) and angle
of maximum turn can be varied in the model but have little effect on mate-
finding or catch results (Byers, 1991, 1993). When insects impact the area's
boundaries they rebound at a random angle. Initial angular directions and
positions of individuals are chosen at random. The `insects' move a step at a
time up to the number of moves determined by the test duration and the speed
of flight, or until caught by a trap. Insects are removed from the simulation
if caught and the percentage of the initial population caught at the end of
the test, or the time observed to catch all the insects, is recorded at the
end.
Iterative equations (discussed subsequently) were derived that yield mean
values identical, within statistical variation, to the results of simulations
(Byers, 1993). The simulation model was used to validate selected results of
the iterative equations. However, since the simulation model is time-consuming
compared to the iterative equations (more than a million times slower with
some parameters), only the iterative equations were used to generate results
presented here. The simulation and iterative programs are available from the
author (send IBM-compatible disk).
Gries et al. (1989) used a density of 500 spruce trees/ha in their
simulations of host-finding in I. typographus. However, the
relationship between trunk diameter and density must be considered in the
models presented here. Linear regression of data from Magnussen (1986) in his
figure 3, relating density of Norway spruce (300 to 900 trees/ha) and trunk
diameter, shows that trees have an average diameter of about 0.29 m at 500
trees/ha. Thus, in all models (except where diameter was varied) the tree
diameter was set at 0.3 m in a forest of density 500 trees/ha. Ips
typographus beetles fly at about 1.9 m/s (Gries et al., 1989) or 2 m/s
(Byers et al., 1989), the latter value was used in the models. Populations of
flying I. typographus probably can vary up to some hundreds of
thousands per km2, but 9000/km2 were estimated in one study (Byers et al.,
1989) and used in the models except when density was varied.
3. RESULTS
The first question that must be answered is how far would a beetle be
expected to fly before being intercepted by the trunk of a tree in the above
forest? If a beetle is released at random in the forest then the average
expected distance D that a beetle would fly before striking a tree is
given by:
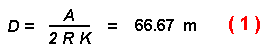
This distance of 66.7 m is also what a beetle would be expected to travel when
flying away from one tree until intercepting another. This distance should be
much larger than the expected average distance to the nearest tree (2.34 m)
for a forest of Poisson distribution (Clark and Evans, 1954) since beetles can
fly in any direction. The average flight distance beetles would fly before
being intercepted by another tree can be calculated from equation (1) for
different numbers of trees (K) per km2 and different tree radii (Figure
1).
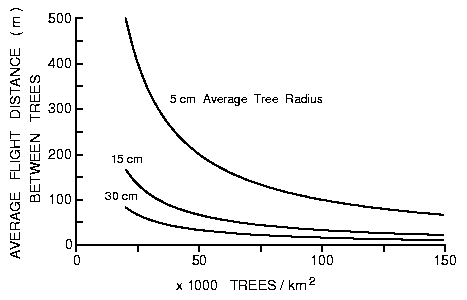
Fig. 1. Expected average flight distance a bark beetle would fly before
striking a tree depending on the density of trees at three specific diameters.
If beetles fly forward with a random component until nearly striking a
trunk they might be able, at best, to instantly determine the susceptibility
of a tree without the need to land. In this case, the flight speed would be
the maximum of 2 m/s while searching for hosts. More likely, beetles must land
on a tree for a period of time, possibly to bore through the outer bark,
before they can determine the resistance of a potential host (Elkinton and
Wood, 1980). This "testing time" per tree is not known for any species of bark
beetle, but can be hypothesized to range up to several hours. The effective
speed (S) of a beetle would then be less than the flight speed
(Sf) as found from the following equation:
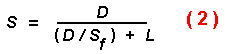
where L is the landing time on each tree.
The time required for the first beetle of a population to find a tree is
next considered. Let R = radius of a tree trunk in m (e.g. 0.15),
K = number of trees (e.g. 50000), T = time, S = flight
speed in m/s (e.g. 2), N = number of initial insects (e.g. 9000), and
A = area in mý (e.g. 1,000,000), then the first beetle intercepts a
tree in the forest after:
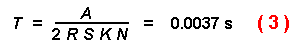
This time is short indeed since it represents the time before the first of any
beetles in the population contacts a tree.
The iterative equations that follow were derived by summing the times
required for each individual of the population to intercept a tree trunk.
K can also represent the number of susceptible trees rather than all
trees, and would then be a small percentage of the number of trees in the
forest. How many susceptible trees is a matter of conjecture and depends on
forest conditions, but was varied in the models from 1 to 1000 trees/km2.
Also, the time a beetle can search is ill-defined but could only occur when
the temperature was over 19° C and while the beetle was able to fly
(Annila, 1969; Byers and Löfqvist, 1989). In most models, 8 h was used since
it is known that I. typographus can fly this long on flight mills, and
longer if allowed to feed on spruce and take intermittent flights (Forsse and
Solbreck, 1985; Forsse, 1991). Thus, the time (T) for a certain
proportion (P) of the initial population (N) to find the rare
susceptible trees (K) can be found with the following iterative
equation:
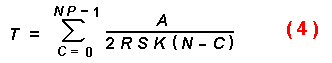
where C is the catch or encounters with susceptible trees. The
relationship between hours searching by 9000 beetles at an effective maximum
speed of 2 m/s (no landing per tree) in a 1 km2 area for several densities of
susceptible trees (1 to 1000/km2) where all 50000 trees have a radius of 0.15
m is shown in Figure 2 (from equation 4). All beetles find the susceptible
trees (at 1000/km2) within a few hours, while 15.9% of the population find
susceptible hosts in 8 h if there are only 10 of these trees per km2 (0.02% of all
trees).
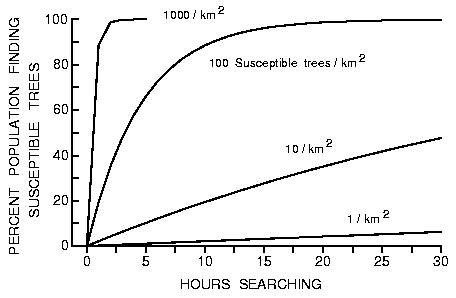
Fig. 2. Effect of time (hours searching) on the percent of the simulated bark
beetle population finding susceptible trees (1 to 1000/km2) in a forest of
50000 trees/km2. All trees have a radius of 0.15 m and beetles are assumed to
fly at 2 m/s (0 s landing per intercepted tree).
The length of time a beetle tested a tree they landed upon would affect
the effective speed (S) during an 8 h search and consequently the
proportion of the population finding susceptible hosts (Figure 3, using
equations 2 and 4).
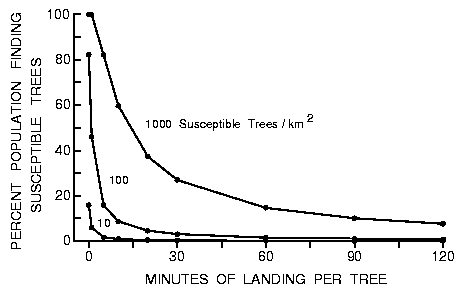
Fig. 3. Effect of varying the time a beetle lands on an intercepted tree to
test its susceptibility on the percent of the population of 9000 searching
beetles that find 10 to 1000 susceptible trees in a forest of 50000 trees each
of 0.15 m radius during an 8 h period in a 1 km2 area (beetles fly at 2 m/s
between landings).
The number of beetles from the population of 9000 searching in the forest that
find susceptible hosts in 8 h depends on the density of these hosts (e.g., 1
or 10/km2) and the time spent testing trees landed upon (Figure 4). For
example, if beetles on average test trees they land upon for 20 min and there
are 10 trees/km2 available for colonization, then 42 beetles find these trees,
or an average of 4 beetles per tree (Figure 4).

Fig. 4. Effect of varying the time a beetle lands on an intercepted tree to
test its susceptibility on the number of beetles in a population of 9000
beetles that find 1 or 10 susceptible trees in a forest of 50000 trees each of
0.15 m radius during an 8 h period in a 1 km2 area (beetles fly at 2 m/s
between landings).
These beetles, by definition, are able to attack the susceptible hosts and
produce aggregation pheromone. The pheromone attracts beetles from several
meters and would, in effect, increase the radius of the tree, causing more
beetles to find these trees.
The entire population of beetles flying at maximum effective speed (2
m/s) would find ten widely scattered susceptible hosts in 8 h if aggregation
pheromone from beetles in these trees attracted beetles within an effective
radius of 15 m (Figure 5). If the effective speed of beetles was only 0.0092
m/s due to landing for 2 h on each tree tested, then 7.66% or 689 beetles
(69/tree) locate susceptible hosts in 8 h (Figure 5).
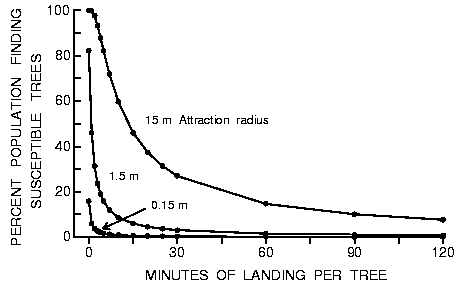
Fig. 5. Effect of varying the time a beetle lands on an intercepted tree to
test its susceptibility on the percent of the population of 9000 searching
beetles that find 10 susceptible trees in forests of 50000 trees of three
different diameters during an 8 h period in a 1 km2 area (beetles fly at 2 m/s
between landings).
This indicates that when the few susceptible trees are located by a small
proportion of the population and they produce pheromone, a much larger
proportion of the population can then find these effectively larger trees.
However, the process is gradual since each beetle that finds the tree and
produces pheromone only increases the tree's effective radius a small amount.
This increase in radius increases the rate at which beetles arrive on the tree
which in turn increases the radius and so on.
The effective attraction radius (EAR) is a method for comparing
the relative strengths of semiochemical attractants regardless of the
population density of flying insects (Byers et al., 1989). The EAR
represents a spherical radius (in the simulations a circular radius) that
would be needed by a passive trap (without baits) to catch the same number of
insects as a baited trap (or attractive tree). A synthetic pheromone bait for
I. typographus releasing 50 mg/day methyl butenol and 1 mg/day
cis-verbenol approximates that released by at least 150 males
(Birgersson et al., 1984), and this bait had an EAR of about 2 m (Byers
et al., 1989). Thus, assuming a linear increase in radius (Y) with the number
of beetles attracted (1:1 sex ratio) then Y = X/150 + 0.15 gives a radius of 2
at 300 beetles (Figure 6). Perhaps a more realistic relationship yielding the
same radius of 2 at 300 beetles is Y = 2 ln (X + 1)/ln 300 (Figure 6) since
attraction rates of insects commonly are related logarithmically to pheromone
release rates (Byers and Wood, 1981; Tilden and Bedard, 1985; Byers et al.,
1988).
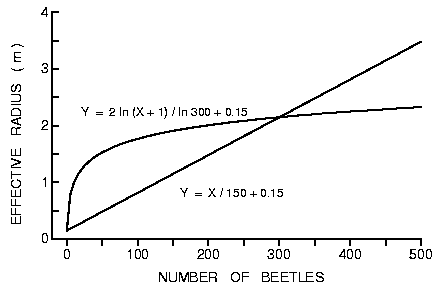
Fig. 6. Theoretical effective radius of susceptible trees under attack by bark
beetles in which aggregation pheromone effectively increases the tree's radius
depending on the number of beetles finding the these trees and producing
pheromone. The logarithmic and linear relationships intersect at an effective
radius of 2 m and 300 beetles (see text for more details).
The dynamic process of host-finding and increase of effective attraction
radius of attacked trees can now be modelled. Equation (4) is used with a
modification for R with P = 1. As each beetle (C) finds a
tree, the average effective radius of susceptible trees with an initial radius
R = 0.15 m increases linearly as in equation (5)
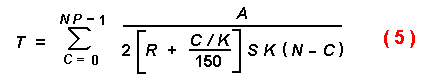
or logarithmically as in equation (6).
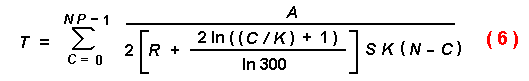
These equations were used to generate the curves shown in Figures 7(a) and
7(b), respectively.
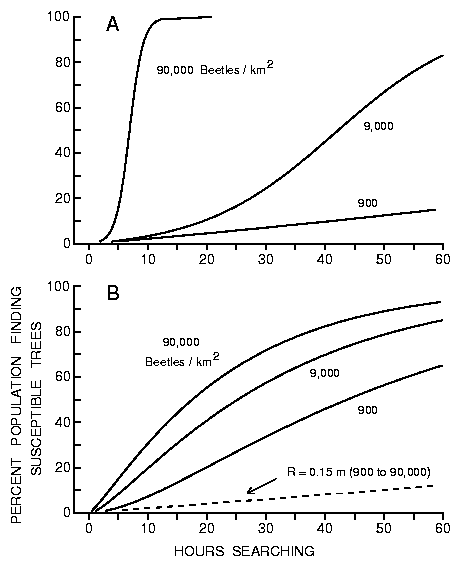
Fig. 7A. Effect of time (hours searching) on the percent of simulated bark
beetle populations (900 to 90,000) finding 10 susceptible trees in a forest of
50000 trees/km2 in which the effective radius of susceptible (attacked) trees
increase linearly (from Figure 6) as beetles find the susceptible trees and
produce pheromone (from equation 5).
Initially as each beetle C finds a susceptible tree, the effective
radius increases and rapidly allows more beetles to find these trees, but then
the population density declines gradually reducing the rate of recruitment in
spite of the large effective radius (Figures 7(a), (b)).
Fig. 7B. Effect of time (hours searching) on the percent of simulated bark
beetle populations (900 to 90,000) finding 10 susceptible trees in a forest of
50000 trees/km2 in which the effective radius of susceptible (attacked) trees
increase logarithmically (from Figure 6) as beetles find the susceptible trees
and produce pheromone (from equation 6). Beetles are assumed to fly at 2 m/s
but land for 5 min. on intercepted trees to test their susceptibility.
The rates at which the effective radii are increased depend on the population
density, at higher densities the radii are increased rapidly and catch
proportionately more of the population (Figures 7(a), (b)). When the beetles
can not produce pheromone and thus do not change the effective radius of a
host tree, then the percent of the population finding trees is not affected by
the population density (dashed line in Figure 7(b)).
4. DISCUSSION
Gries et al. (1989) modelled the host-finding of I. typographus in
a simulated spruce forest of 1 km2 of 50000 "trees" with 3000 to 16500
susceptible hosts and from 20000 to 100000 flying beetles (values standardized
for comparison here). Four sub-models were tested in which "beetles" had
flight paths that were either random or upwind, and with or without the
effective size of "trees" enlarged to simulate primary attraction. They
concluded that for random search to be effective in locating susceptible trees
and maintaining population levels, a longer flight dispersal is required than
is possible (assuming a maximum 3 km flight dispersal). They gave the
impression that an "upwind" flight strategy is what beetles in nature should
employ since simulated beetles located most host trees in the upwind model.
However, this result must depend on the wind speed since a wind speed matching
the flight capacity would not allow upwind search to proceed. Previous models
indicate that a straight path would yield the maximum interception rate of
susceptible hosts per distance travelled, but that the rate of directional
changes (randomness) has surprisingly little effect on the rate of encounters
with hosts (Byers, 1991). In fact, beetles could cover more area with less
energy expense by flying downwind, especially at higher wind speeds, until
perceiving pheromone, whereupon they would fly upwind using anemotactic
mechanisms (Byers, 1995). Bark beetles, including I. typographus,
generally fly away from release sources in all directions unless winds are
strong when they appear to passively drift with the wind (Meyer and Norris,
1973; Helland et al., 1984; Byers et al., 1989; Salom and McClean, 1991;
Zolubas and Byers, 1995).
The results here suggest that bark beetles can find hosts at much lower
population densities of beetles (9000) and also at lower densities of
susceptible trees (1 to 1000) than those tested by Gries et al. (1989) above.
The reason beetles find the few susceptible trees in my models even at endemic
levels of beetles, as they must do in nature, is that the few pioneer beetles
that intercept susceptible trees through "random" flight recruit other beetles
that would not otherwise find these trees, thereby enlarging the effective
radius of the attacked tree by means of aggregation pheromone.
Both the model here and that of Gries et al. (1989) consider the distance
beetles might disperse. Gries et al. (1989) measured the weight loss of
beetles until they choose to feed on a fresh spruce log and compared this
weight loss to that of flying beetles in order to estimate a maximum flight
capacity of 3 km (before choosing to feed). However, this distance of 3 km
reflects the distance a beetle might disperse until feeling "hungry" rather
than a maximum dispersal ability. For example, Jactel and Gaillard (1991) flew
I. sexdentatus on rotary flight mills connected electronically to a
computer. They found that 50% of these beetles could fly more than 20 km and
10% more than 45 km based on about 50 interrupted flights. In another study
where I. typographus were placed on a flight mills, the longest
continuous fight was 6 h and 20 min (Forsse and Solbreck, 1985). This would
mean that a few I. typographus could fly up to 45.6 km at a speed of 2
m/s without resting (Byers et al., 1989). In the models here, flights were a
maximum of 8 h (Figs. 2-5) depending on landing time, or ranged up to 60 h
(Fig. 7A) with 300 s landing per tree. It might be possible for beetles to
replenish their energy and fat reserves for further flight by feeding on host
phloem, and they can live for up to 2-3 weeks during the spring dispersal
period while attempting flights in a cage on the forest floor (Byers and
Löfqvist, 1989).
One major uncertainty of the present models is whether, and for how long,
beetles land on a tree to determine its susceptibility. This landing time per
intercepted tree was varied from 0 to 2 h (Fig. 3 and
Fig. 5). Elkinton and Wood (1980) found that I. paraconfusus apparently
could not discriminate the host, ponderosa pine, from a non-host, white fir,
until after penetrating the outer bark and contacting the phloem, whereupon
the beetles left the boring sites in white fir. The time to penetrate white
fir bark was not measured precisely but appeared to take several hours. In
contrast, this beetle appears to reject another native non-host, incense
cedar, much more rapidly since bark was rarely penetrated. If beetles take as
long as a day to determine the suitability of a host tree this would
significantly alter the finding-rates suggested here. However, beetles that
had to leave relatively resistant trees might be able to feed long enough to
renew their flight dispersal ability. Using the same parameters as in Fig. 2,
but landing times of 24 h, then 9 beetles can find 9 of 100 susceptible trees
in 12 h, and then begin to produce pheromone. With the model of
logarithmically increasing radii (Fig. 7B), 10% of 90,000 beetles that landed
24 h per tree would find 1000 susceptible trees in 31.3 h.
The model here assumes beetles fly equally in all areas. However, many
bark beetle species in the spring fly along forest edges (next to a clearcut)
when temperatures are just above the threshold for flight (Forsse and Solbreck
1985, unpublished data). This behavior may be due mostly to temperature
conditions, but also could be due to the fact that more susceptible trees are
found along the clearings due to solar and wind stress. Later in the flight
period, when temperatures are optimal, beetles appear to fly within the forest
as well (Forsse and Solbreck 1985; unpublished data). The boundary in the
model should not affect conclusions concerning nature, assuming adjacent
forest areas have similar numbers of susceptible trees and beetles - so that
immigration and emigration are equal.
The model suggests that long-range primary attraction to host tree
volatiles is not necessary for host finding and selection in many species of
bark beetle, especially species that have a potent aggregation pheromone. For
example, the attraction of the important pest species, D. brevicomis,
I. paraconfusus and I. typographus to host volatiles is very
weak or non-existent (Moeck et al., 1981; Schlyter et al., 1987), while they
are attracted over several meters to aggregation pheromone (Byers, 1983; Byers
et al., 1989). Other bark beetles such as D. ponderosae and Scolytus
multistriatus are attracted to either aggregation pheromone or host
volatiles (Gore et al., 1977; Gara et al., 1984).
Some bark beetles such as Pseudohylesinus nebulosus, T.
piniperda and probably Hylurgops palliatus do not appear to use an
aggregation pheromone (Ryker and Oester, 1982; Byers et al., 1985; Klimetzek
et al., 1986; Byers, 1992). In these species there may be little selection
pressure to evolve aggregation pheromones since they respond over several
meters to plant volatiles from wounds and decay that reveal the location of a
susceptible host. Evolution of an olfactory response to host volatiles is more
probable in species with normally low population densities and widely
dispersed host plants.
The host-plant finding model here can be regarded as a predator-prey
encounter model where the predator remains stationary; for example, ant lions
in their conical pits feeding on ants. Speakman (1986) derived equations for
the optimal search speed of a predator when searching for stationary prey of a
specified density. His equation for the time spent searching is of interest
here and was equal to the reciprocal of the encounter rate. This rate was
equal to the search speed multiplied by D, where D was the
density of prey along the search path. DeVita et al. (1982) express an
encounter rate between individuals of a population moving at random with a
specified speed as:
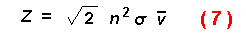
where n is animal density, sigma the size, and v the mean speed. These
encounter rate equations assume a constant density of animals which is not the
case in the present model since beetles stay when finding a suitable host
tree.
Another related model is the functional response equation of Holling
(1959). This well known equation defines the encounter rate between predator
and prey as the amount of area covered in a unit of time multiplied by the
prey density and total search time. His equation does not account for
depletion of the prey during the period. Royama (1971) and Rogers (1972)
modified the Holling equation to account for exponential decay of prey
densities over time in the `random predator equation':
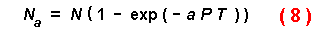
where Na is the number of prey eaten during total time
T at an attack coefficient a and predator density P and
initial prey number of N (with no handling time). This equation uses
the attack coefficient (area of search per unit time) to address the effective
radius and speed of prey or predators. Their assumption of exponential decay
of prey densities agrees with my equations, for example, plotting the decline
in population level with time for the parameters with 100 susceptible trees
(Fig. 2) yields data fitting perfectly an exponential equation (Y =
101.3 exp[-0.218X]). In fact, equation (8) can be used to answer the
same questions addressed here when the attack coefficient is made equal to
2RS.
The iterative equations with appropriate modifications can be used to
describe many kinds of encounters and functional responses in mating and
predator-prey systems and thus could be useful as sub-models in population
dynamic models of greater complexity.
JOHN A. BYERS
Department of Plant Protection, Chemical Ecology
Swedish University of Agricultural Sciences
S-230 53 Alnarp, Sweden
|
Present address: 
|
|---|
ACKNOWLEDGEMENTS
Helpful reviews of the manuscript were given by my colleagues Fredrik
Schlyter and Olle Anderbrant. This work was supported by a grant from the
Swedish Agricultural and Forest Research Council, SJFR.
REFERENCES
Annila, E., 1969. Influence of temperature upon the development and voltinism
of Ips typographus L. (Coleoptera, Scolytidae). Ann. Zool. Fenn.,
6: 161-207.
Berryman, A.A. and Ashraf, M., 1970. Effects of Abies grandis resin on
the attack behavior and brood survival of Scolytus ventralis
(Coleoptera: Scolytidae). Can. Entomol., 102: 1229-36.
Birgersson, G., Schlyter, F., Löfqvist, J. and Bergström, G., 1984.
Quantitative variation of pheromone components in the spruce bark beetle
Ips typographus from different attack phases. J. Chem. Ecol.,
10: 1029-1055.
Byers, J.A., 1983. Sex-specific responses to aggregation pheromone: Regulation
of colonization density in the bark beetle Ips paraconfusus. J.
Chem. Ecol., 9: 129-142.
Byers, J.A., 1991. Simulation of mate-finding behaviour of pine shoot beetles,
Tomicus piniperda. Anim. Behav., 41: 649-660.
Byers, J.A., 1992. Attraction of bark beetles, Tomicus piniperda,
Hylurgops palliatus, and Trypodendron domesticum and
other insects to short-chain alcohols and monoterpenes. J. Chem.
Ecol., 18: 2385-2402.
Byers, J.A., 1993. Simulation and equation models of insect population control
by pheromone-baited traps. J. Chem. Ecol., 19: 1939-1956.
Byers, J.A., 1995. Host tree chemistry affecting colonization in bark beetles.
In: R. T. Cardé and W. J. Bell (Editors), Chemical Ecology of Insects II.
Chapman and Hall, New York, pp. 154-213.
Byers, J.A. and Löfqvist, J., 1989. Flight initiation and survival in the bark
beetle Ips typographus (Coleoptera: Scolytidae) during the spring
dispersal. Holarct. Ecol., 12: 432-440.
Byers, J.A. and Wood, D.L., 1981. Interspecific effects of pheromones on the
attraction of the bark beetles, Dendroctonus brevicomis and Ips
paraconfusus in the laboratory. J. Chem. Ecol., 7: 9-18.
Byers, J.A., Anderbrant, O. and Löfqvist, J., 1989. Effective attraction
radius: A method for comparing species attractants and determining
densities of flying insects. J. Chem. Ecol., 15: 749-765.
Byers, J.A., Birgersson, G., Löfqvist, J. and Bergström, G., 1988. Synergistic
pheromones and monoterpenes enable aggregation and host recognition by a
bark beetle, Pityogenes chalcographus. Naturwissenschaften, 75:
153-155.
Byers, J.A., Lanne, B.S., Löfqvist, J., Schlyter, F. and Bergström, G., 1985.
Olfactory recognition of host-tree susceptibility by pine shoot beetles.
Naturwissenschaften, 72: 324-326.
Clark, P.J. and Evans, F.C., 1954. Distance to nearest neighbor as a measure
of spatial relationships in populations. Ecology, 35: 445-53.
DeVita, J., Kelly, D. and Payne, S., 1982. Arthropod encounter rate: A null
model based on random motion. Amer. Nat., 119: 499-510.
Elkinton, J.S. and Wood, D.L., 1980. Feeding and boring behavior of the bark
beetle Ips paraconfusus (Coleoptera: Scolytidae) on the bark of a
host and non-host tree species. Can. Entomol., 112: 797-809.
Forsse, E., 1991. Flight propensity and diapause incidence in five populations
of the bark beetle Ips typographus in Scandinavia. Entomol. Exp.
Appl., 61: 53-58.
Forsse, E. and Solbreck, C., 1985. Migration in the bark beetle Ips
typographus duration timing and height of flight. Z. Angew.
Entomol., 100: 47-57.
Gara, R.I., Geiszler, D.R. and Littke, W.R., 1984. Primary attraction of the
mountain pine beetle Dendroctonus ponderosae to lodgepole pine
Pinus contorta in Oregon. Ann. Entomol. Soc. Amer., 77:
333-334.
Gore, W.E., Pearce, G.T., Lanier, G.N., Simeone, J.B., Silverstein, R.M.,
Peacock, J.W. and Cuthbert, R.A., 1977. Aggregation attractant of the
European elm bark beetle, Scolytus multistriatus. Production of
individual components and related aggregation behavior. J. Chem.
Ecol., 3: 429-446.
Graham, K., 1968. Anaerobic induction of primary chemical attractancy for
ambrosia beetles. Can. J. Zool., 46: 905-908.
Gries, G., Nolte, R. and Sanders, W., 1989. Computer simulated host selection
in Ips typographus. Entomol. Exp. Appl., 53: 211-217.
Helland, I.S., Hoff, J.M. and Anderbrant, O., 1984. Attraction of bark beetles
(Coleoptera: Scolytidae) to a pheromone trap: experiment and mathematical
models. J. Chem. Ecol., 10: 723-52.
Hodges, J.D., Nebeker, T.E., DeAngelis, J.D., Karr, B.L. and Blanche, C.A.,
1985. Host resistance and mortality: A hypothesis based on the southern
pine beetle-microorganism-host interactions. Bull. Entomol. Soc. Am., 31:
31-5.
Holling, C.S., 1959. Some characteristics of simple types of predation and
parasitism. Can. Entomol., 91: 385-398.
Hynum, B.G. and Berryman, A.A., 1980. Dendroctonus ponderosae
(Coleoptera: Scolytidae) pre-aggregation landing and gallery
initiation on lodgepole pine. Can. Entomol., 112: 185-192.
Jactel, H. and Gaillard, J. 1991. A preliminary study of the dispersal
potential of Ips sexdentatus Boern (Coleoptera: Scolytidae) with
an automatically recording flight mill. J. Appl. Entomol., 112:
138-145.
Klimetzek, D., Köhler, J., Vité, J.P. and Kohnle, U., 1986. Dosage response to
ethanol mediates host selection by `secondary' bark beetles.
Naturwissenschaften, 73: 270-272.
Magnussen, S., 1986. Diameter distributions in Picea abies described by
the Weibull model. Scand. J. For. Res., 1: 493-502.
Meyer, H.J. and Norris, D.M., 1973. A mathematical relation to describe the
influence of wind on the initial flight dispersal of Scolytus
multistriatus (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am.,
66: 505- 508.
Miller, J.M. and Keen, F.P., 1960. Biology and control of the western pine
beetle. U.S. Dept. Agric. Misc. Publ. No. 800.
Moeck, H.A., 1970. Ethanol as the primary attractant for the ambrosia beetle
Trypodendron lineatum (Coleoptera: Scolytidae). Can. Entomol.,
102: 985-95.
Moeck, H.A., 1981. Ethanol induces attack on trees by spruce beetles
Dendroctonus rufipennis (Coleoptera: Scolytidae). Can. Entomol.,
113: 939-942.
Moeck, H.A., Wood, D.L. and Lindahl, K.Q. Jr., 1981. Host selection behavior
of bark beetles (Coleoptera: Scolytidae) attacking Pinus
ponderosa, with special emphasis on the western pine beetle,
Dendroctonus brevicomis. J. Chem. Ecol., 7: 49-83.
Rogers, D., 1972. Random search and insect population models. J. Anim. Ecol.,
41: 369-383.
Royama, T., 1971. A comparative study of models for predation and parasitism.
Res. Pop. Ecol. 1 (Suppl.): 1-99.
Ryker, L.C. and Oester, P.T., 1982. Pseudohylesinus nebulosus
(Coleoptera: Scolytidae) aggregation by primary attraction. Z.
Angew. Entomol., 94: 377-382.
Salom, S.M. and Mclean, J.A., 1991. environmental influences on dispersal of
Trypodendron lineatum (Coleoptera: Scolytidae). Environ.
Entomol., 20: 565-576.
Schlyter, F., Birgersson, G., Byers, J.A., Löfqvist, J. and Bergström, G.,
1987. Field response of the spruce bark beetle, Ips typographus,
to aggregation pheromone candidates. J. Chem. Ecol., 13:
701-716.
Skellam, J.G., 1973. The formulation and interpretation of mathematical models
of diffusionary processes in population biology. In: M. S. Bartlett and
R. W. Hiorns (Editors), The Mathematical Theory of the Dynamics of
Biological Populations. Academic Press, London, pp. 63-85.
Smith, R.H., 1961. The fumigant toxicity of three pine resins to
Dendroctonus brevicomis and D. jeffrei. J. Econ.
Entomol., 54: 365-369.
Speakman, J.R., 1986. The optimum search speed of terrestrial predators when
feeding on sedentary prey: a predictive model. J. Theor. Biol., 122: 401-
407.
Struble, G.R. and Hall, R.C., 1955. The California five-spined engraver. Its
biology and control. USDA Cir. No. 964:21p.
Tilden, P.E. and Bedard, W.D., 1985. Field response of Dendroctonus
brevicomis to exo-brevicomin, frontalin, and myrcene released
at two proportions and three levels. J. Chem. Ecol., 11: 757-66.
Witanachchi, J.P. and Morgan, F.D., 1981. Behavior of the bark beetle Ips
grandicollis during host selection. Physiol. Entomol., 6:
219-224.
Wood, D.L. 1982. The role of pheromones, kairomones, and allomones in the host
selection and colonization behavior of bark beetles. Ann. Rev. Entomol.,
27: 411-46.
Zolubas, P. and Byers, J.A., 1995. Recapture of dispersing bark beetles,
Ips typographus L. (Col., Scolytidae) in pheromone-baited
traps: regression models. J. Appl. Entomol. 119:285-289.
John Byers has a Ph.D. in entomology from the University of California at
Berkeley. He currently is a högskolelektor (associate professor) in the
Department of Plant Protection, Chemical Ecology, Swedish University of
Agricultural Sciences, S-230 53 Alnarp, Sweden. His interests include chemical
ecology of bark beetles and computer simulation of ecological and behavioral
mechanisms.
UPDATES and General scientific software on Internet at:
http://www.wcrl.ars.usda.gov/cec/software.htm
Program software (C) 1996 by:
John A. Byers
Department of Plant Protection
Swedish University of Agricultural Sciences
230 53 Alnarp
SWEDEN

 Abstract
Abstract